2019.04.01.12
Files > Volume 4 > Vol 4 No 1 2019
REVISION/REVIEW
FSH in bovine superovulation
Valeria M. Bautista Vega 1; Silvana P. Jiménez Chávez 2; Catherine D. Meza Franco 2; Thelvia I. Ramos 1; Jorge R. Toledo 2
Available from: http://dx.doi.org/10.21931/RB/2019.04.01.12
ABSTRACT
Bovine follicle stimulating hormone (FSH) is the hormone mainly used for superovulation treatments. It is used so that several secondary follicles can reach a dominant state at the same time and thus, treated cows can release up to ten or more ovules in each zeal, decreasing the generational interval and increasing livestock production. The hormones available in the current market are obtained mostly from pituitary extracts of swine and sheep, and although they are widely used. Several negative aspects have been reported, implying high risks of contamination with pathogens, contamination with other hormones that interfere with assisted fertilization processes, significant variations between each production batch and the decreased half-life that exhibit FSH leading to excessive handling of donor cows. In this review, we detail some new approaches to overcome these problems, like slow-release FSH formulations that have been developed in order to increase the half-life of FSH and, finally the use of recombinant DNA technology to ensure a pure product.
Key words: FSH, bovine, superovulation, recombinant.
INTRODUCTION
Follicle-stimulating hormone (FSH), produced by the anterior pituitary gland, is a glycoprotein which plays an essential role in reproductive processes1 in several vertebrates2. When the liberation from anterior pituitary occurs, FSH acts in the ovarian granulosa cells and the Sertoli cells in testis3, stimulating folliculogenesis and steroidogenesis in the ovary and spermatogenesis4. FSH, like other glycoprotein hormones, consists of two polypeptide chains (α and β subunit), non-covalently associated. The α subunit, encoded by a single gene, is identical in the amino acid sequence in all gonadotropins within a particular species5, whereas, the biological specificity of these hormones arises from the β subunit which, depending on the type of gonadotropin, is encoded by a different gene (FSHβ, LHβ or CGβ)[i]6. The α subunit of the bovine follicle-stimulating hormone has five intrachain disulfide bridges, while the β subunit, containing 12 cysteine residues7, has six disulfide bridges intrachain8.
Both subunits present post-translational modifications like glycosylation in which sugar moieties like mono- or oligosaccharides9 are transferred from donor molecules to nascent proteins by glycosyltransferases10. The glycans addition is essential for assembly, integrity, secretion and signal transduction in the α-subunit and assembly and secretion in the β-subunit. 1 Two types of glycosylations are present in FSH, N-glycosylations, and O-glycosylations. However, the predominant are of type N5, having two potential N-glycosylation sites in both alpha and beta subunits being of the Asn-Xaa-Thr type11. In bovine FSH, these N-linked oligosaccharides are located at positions N56 and N82 in the α-subunit and N7 and N24 in the β-subunit. Sialylated Asn-linked carbohydrates predominate in bovine FSH 7.
It has been elucidated that exposure of galactose residues, due to lack of glycosylation, on oligosaccharides increases the clearance from plasma 1 due to receptor-mediated endocytosis of asialoglycoproteins by hepatocytes 12. These number of exposed galactose residues is essential in elucidating the FSH half-life1. Some studies reported that FSH with fewer sialylations has a higher receptor binding activity and in vitro bioactivity than the sialylated FSH 13,14. However, the in vivo bioactivity of acidic FSH is 20-higher than the basic FSH15. Despite this, it has been shown that sialic acid plays an important role to prevent rapid clearance of FSH from circulation and it is not critical for receptor binding 7.
Oestrus cycle in cows
Different hormones regulate the estrous cycle: the gonadotropin-releasing hormone (GnRH), which is secreted by the hypothalamus, stimulates the secretion of both follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by Gonadotropic cells of the adenohypophysis16; in addition, by progesterone (P4), estradiol (E2) and inhibin’s in the ovaries; and prostaglandin F2α (PGF) in the uterus 17. In cattle, the oestrus cycle lasts from 18 to 24 days. Two phases are present in the cycle: the luteal phase (14–18 days) and the follicular phase (4–6 days) 18,19. In the luteal phase, the corpus luteum (CL), formed from the collapsed ovulated follicle, is developed from the follicular wall and produces prevalently progesterone stimulated by LH pulses 19. During the follicular phase, final maturation and ovulation of the ovulatory follicle occur. Additionally, the progesterone levels decrease significantly due to the declining of corpus luteum function 18.
During the entire cycle, there are two or three waves of ovarian follicle growth (Fig. 1.), for dairy cows and beef cows, respectively, in which occur the emergence of a cohort of 5-20 follicles stimulated by the increase of FSH concentrations. Then, selection and dominance of one follicle occur due to the secretion of inhibins and estradiol (E2) by the growing cohort that decreases FSH concentrations20. This decrease in FSH leads to subordinate follicles regression 21.

Figure 1. Schematic description of the secretion pattern of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and progesterone. Waves of ovarian follicles growth.
Knowledge of the estrous cycle is crucial when discovering new techniques to obtain an increase in livestock production.
Embryo transfer
Embryo transfer (ET) is a technique in which one or more embryos are removed from the reproductive tract of a donor female and then transferred to the lumen of the oviduct or uterus of one or more recipient females 22. It is applied to obtain a maximum number of embryos of a genetically superior animal in the shortest possible time and thus maximizes the genetic potential in a herd obtaining elite females or stallions 23. For ET, different steps must be carried out like: selection of a donor cow, superovulation, insemination, collection and evaluation of embryos, selection and preparation of recipient females, embryo transfer and finally, embryo transplant evaluation 24.
Superovulation is a hormonal treatment that induces follicular growth allowing a more significant number of oocytes to be recovered in the donor bovine female 25, being a pivotal procedure to maximize the number of viable embryos with high capacity to produce pregnancy 26. The basic principle of superovulation is to stimulate follicular development by hormonal treatment and induce ovulation of several follicles simultaneously 27. Also, by natural reproduction, a cow can breed a calf per year, reaching an average of eight to ten calves in its entire life. With an adequate hormonal regimen of superovulation, treated cows can release up to ten or more ovules in each zeal 25, decreasing the generational interval and increasing the genetic potential and production of livestock. 28
Superovulation can be achieved through the application of gonadotropin hormones29, allowing to emulate the natural reproductive processes in order to increase reproductive efficiency. The most commonly used gonadotropins in cattle reproduction include stimulating follicle hormone (FSH) and luteinizing hormone (LH), extracted from the pituitary gland of swine and sheep 29, in addition to equine chorionic gonadotropin (eCG), produced by the endometrial cups of pregnant mares 30.
Common superovulation treatment
In superovulation treatments, exogenous FSH, obtained from porcine and sheep pituitaries 31, is used to rescue secondary follicles from regression so that they could reach a dominant state too 32. The most common protocol for superovulation includes GnRH-induced ovulation of the persistent follicle and follicle wave emergence. Then, the super stimulation starts with the administration of exogenous FSH during four days with twice-daily decreasing doses 33.
As the FSH used in treatments for superovulation comes from pituitary of animals, this may be contaminated with traces of other hormones, leading to variations in oocyte quality and quantity. Several authors showed that FSH products with low LH levels improve the ovulation rate, fertilization rate and embryo quality 34,35. Kelly et al. (1997) 36 evaluated the follicular growth pattern and the performance of embryos in cattle after superovulation with two preparations of FSH differing in LH content (Serovet; FSH:LH ratio 1:1 y Vetrepharm; low LH). A higher number of ovulations was observed in cows treated with Serovet compared with Vetrepharm, this increase was mainly the result of a higher number of unfertilized ova and degenerated embryos unfit for transfer. Therefore, FSH with high purity or reduced LH content has become the method of choice for commercial use for the production of superovulatory cows.
On the other hand, it has been seen that the repeated use of exogenous hormones to induce superovulation in different species could induce a humoral immune response 37. eCG began to be used, as a superovulatory treatment in cows 31. Because of its long half-life being of 40 hours and its persistence in the circulation of up to 10 days, it is necessary a single dose for the superovulatory response 38. Its persistence in circulation is due to a large number of glycosylations sites in its structure, being the most glycosylated glycoprotein, covering 45% of its molecular weight 39. However, one study showed that treatment with eCG in bovines induced a humoral immune response at repeated doses 40. Therefore, the humoral response generated makes these treatments ineffective over time due to the production of neutralizing antibodies, thus reducing the super stimulating response.
There are several problems also associated with prolonged stimulation of the eCG including the continuous stimulation of the ovaries, follicles without ovulation, abnormal endocrine profiles and reduction of embryo quality 41. Besides, a better superovulatory response has been established after treatment with FSH compared with eCG 42. Additionally, due to the high molecular weight of eCG, a more significant percentage of ovarian cysts (40%) appeared about the treatment of FSH (8%) 43. The leading cause of economic losses and reproductive problems is attributed to the presence of these cysts, it also causes abnormal hormonal profiles and a low embryo quality 44.
The twice-daily administration of FSH, because of its short half-life being five days only 45, leads to several problems in the field including excessive handling of donor cows causing stress and decreased superovulatory response 46. Recently, there have been many alternative treatments to try to avoid these problems, for example, the application of slow-release FSH formulations 45. This could be achieved by mixing FSH with polyvinylpyrrolidone (PVP) which maintains a high concentration of FSH in blood enough to stimulate the development of multiple follicles47. However, this treatment has had variable results being unable to induce superovulation in some cases 48. Another alternative is the use of hyaluronan, a glycosaminoglycan, used in a 2% solution to dilute FSH, having the same results with the traditional two-day dose of FSH. However, it is difficult to mix FSH with hyaluronan because of its viscous state leading to problems in the field, and a more diluted solution decreases its effectiveness 49. Another alternative is the use of aluminum hydroxide gel, a vaccine adjuvant, to induce superovulation in cattle 50. However, the use of adjuvants could cause the development of antibodies against FSH 49.
Due to these problems such as variability, immunogenic effects, and inconvenient formulations, other alternatives should be sought to produce superovulation hormones on a larger scale, which are safe for livestock and at a lower cost.
Recombinant DNA technology
The technology of recombinant DNA has allowed building a variety of hormonal analogs with different biological characteristics 51. It has been verified that the construction of recombinant FSH reduces the variation observed in the different types of FSH derived from pituitary gland 31,52. Also, with the recombinant bovine follicle-stimulating hormone, possible risks of immunogenicity are avoided, also resulting in higher purity and less variability product concerning animal extracted hormones.
Today, there are several expression systems for the production of large-scale recombinant proteins, which include expression in E. coli bacteria, baculovirus-mediated insect cells, yeast, and several systems in mammals 53. Expression systems using mammalian cells have a superior capacity to produce biologically active and lower cost proteins 54. In addition, these expression systems are used for the production of recombinant proteins when complex post-translational modifications are necessary for their bioactivity 55, as is the case of FSH, with several N-glycosylation sites, which increase the solubility and stability of the proteins, facilitate its adequate, its appropriate charge and the formation of disulfide bridges 31.
Several researchers have developed the recombinant bovine follicle stimulating a hormone in different expression systems such as yeast 8,56, mammalian cell lines 57,58, insect cells 59, plants 60, mammary gland of mice 61 and rabbit mammary gland 62 (Table 1). Chinese hamster ovary (CHO) cells have been chosen preferably to produce recombinant FSH at a commercial level, especially for those that require posttranslational modifications. However, the culture of mammalian cells is expensive due to the use of rich growing media and supplements, besides its slow-growing 63.
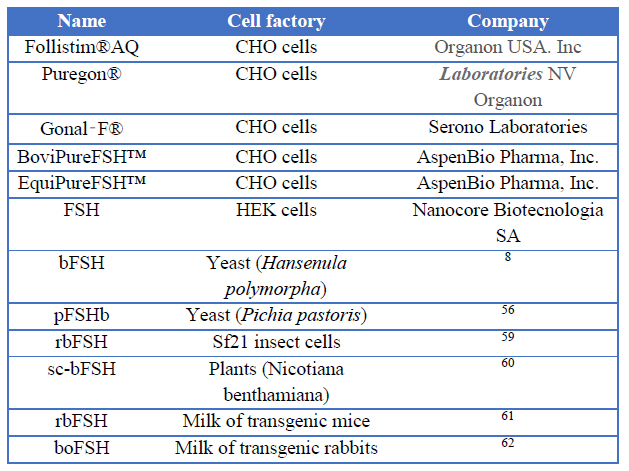
Table 1. Expression systems of recombinant bovine FSH
Many laboratories and pharmaceutical companies have been able to produce a variety of therapeutic proteins in mammals as expression systems, including cows, pigs, sheep, goats and rabbits 64,65. These recombinant proteins are produced from body fluids of animals like milk, egg white, blood, urine and seminal plasma 66.
In recent years, researchers have focused on the mammary gland as an expression system of recombinant proteins, has several advantages. With the direct in vivo transduction of mammary gland, the desired protein is secreted to the milk, having relatively easy purification steps 67. Large volumes of milk could be easily collected 66, depending on the species (Table 2), so large amounts of protein could be obtained 68. The proteins obtained in this expression system have appropriate post-translational processing resulting in proper biological activity 69. Also, these proteins can be used for a long period given its low immunogenicity.70
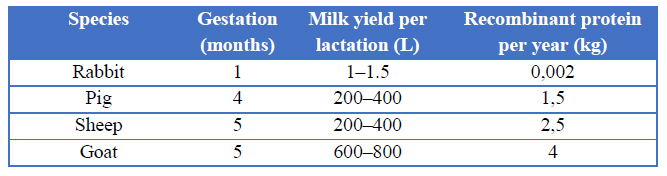
Table 2. Milk expression systems in different species
CONCLUSION
The bovine FSH is of great importance in the production of cattle having a high economic implication. The aim of the application of pure FSH in superovulation protocols is to prevent the variations in oocyte quality and quantity.
Several investigators have used different expression systems to produce pure recombinant hormones, including plants, insect cells, yeasts, bacteria, and mammary gland. The development of proteins in the milk of animals could generate high quality and cost-effective products, preserving the animal wellbeing. The production of recombinant proteins has offered a safe, efficient and economical way to overcome the need for biopharmaceutical products.
The developing of FSH is still a challenge in the scientific world; many expression systems could be tested for better yields. FSH variants of high purity, efficiency and stability can be used in cattle to obtain a higher number of ovules and the subsequent production of embryos of a selected cow and thus reduce the generational time and increase the genetic potential of their offspring.
REFERENCES
1 Ulloa-Aguirre a, Timossi C, Damián-Matsumura P, Dias J a. Role of glycosylation in function of follicle-stimulating hormone. Endocrine 1999; 11: 205–215.
2 De Loof A, Baggerman G, Breuer M, Claeys I, Cerstiaens A, Clynen E et al. Gonadotropins in insects: An overview. Arch Insect Biochem Physiol 2001; 47: 129–138.
3 Touyz RM, Jiang L, Ram Sairam M. Follicle-Stimulating Hormone Mediated Calcium Signaling by the Alternatively Spliced Growth Factor Type I Receptor1. Biol Reprod 2000; 62: 1067–1074.
4 Santi D, Potì F, Simoni M, Casarini L. Pharmacogenetics of G-protein-coupled receptors variants: FSH receptor and infertility treatment. Best Pract Res Clin Endocrinol Metab 2018; 32: 189–200.
5 Mullen M, Cooke D, Crow M. Structural and Functional Roles of FSH and LH as Glycoproteins Regulating Reproduction in Mammalian Species. IntechOpen 2013; Chapter 8.
6 Cahoreau C, Klett D, Combarnous Y. Structure–Function Relationships of Glycoprotein Hormones and Their Subunits’ Ancestors. Front Endocrinol (Lausanne) 2015; 6: 26.
7 Mullen MP, Cooke DJ, Crow M a. Structural and Functional Roles of FSH and LH as Glycoproteins Regulating Reproduction in Mammalian Species. Gonadotropin 2013; : 155–180.
8 Qian W, Liu Y, Zhang C, Niu Z, Song H, Qiu B. Expression of bovine follicle-stimulating hormone subunits in a Hansenula polymorpha expression system increases the secretion and bioactivity in vivo. Protein Expr Purif 2009; 68: 183–189.
9 Roth Z, Yehezkel G, Khalaila I. Identification and Quantification of Protein Glycosylation. Int J Carbohydr Chem 2012; 2012: 1–10.
10 Corfield A. Eukaryotic protein glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol 2017; 147: 119–147.
11 T Rajendra Kumar JSD. Naturally Occurring Follicle-Stimulating Hormone Glycosylation Variants. J Glycomics Lipidomics 2014; 04: 1–10.
12 Springer AD, Dowdy SF. Leading the Way for Delivery of RNAi Therapeutics. 2018; 28: 1–10.
13 Ulloa-Aguirre A, Timossi C. Structure-function relationship of follicle-stimulating hormone and its receptor. Hum Reprod Updat 1998; 4: 260–283.
14 Aggarwal BB, Papkoff H. Relationship of sialic acid residues to in vitro biological and immunological activities of equine gonadotropins. Biol Reprod 1981; 24: 1082–7.
15 De Leeuw R, Mulders J, Voortman G, Rombout F, Damm J, Kloosterboer L. Structure-function relationship of recombinant follicle stimulating hormone (Puregon®). Mol Hum Reprod 1996; 2: 361–369.
16 Kadokawa H, Pandey K, Nahar A, Nakamura U, Rudolf FO. Gonadotropin-releasing hormone (GnRH) receptors of cattle aggregate on the surface of gonadotrophs and are increased by elevated GnRH concentrations. Anim Reprod Sci 2014; 150: 84–95.
17 Rahman ANMA. Hormonal Changes in the Uterus During Pregnancy - Lessons from the Ewe: A Review. J Agric & Rural Dev 2008; 4: 7.
18 Forde N, Beltman ME, Lonergan P, Diskin M, Roche JF, Crowe MA. Oestrous cycles in Bos taurus cattle. Anim Reprod Sci 2011; 124: 163–169.
19 Tomac J, Cekinovć D, Arapović J. Biology of the corpus luteum. Period Biol 2011; 113: 43–49.
20 Scully S, Evans ACO, Duffy P, Crowe MA. Characterization of follicle and CL development in beef heifers using high resolution three-dimensional ultrasonography. Theriogenology 2014; 81: 407–418.
21 Mapletoft RJ, Bó GA. Innovative strategies for superovulation in cattle. 2013; : 174–179.
22 Genzebu D. A Review of Embryo Transfer Technology in Cattle. Glob J Anim Sci Res 2015; 2: 120–126.
23 Patel D, Haque N, Patel G, Chaudhari A, Madhavatar M, Bhalakiya N et al. Implication of Embryo Transfer Technology in Livestock Productivity. IntJCurrMicrobiolAppSci 2018; 7: 1498–1510.
24 Hopper RM. Bovine Reproduction. Wiley, 2014https://books.google.com.ec/books?id=XpM_BAAAQBAJ.
25 Luo C, Zuñiga J, Edison E, Palla S, Dong W, Parker-Thornburg J. Superovulation Strategies for 6 Commonly Used Mouse Strains. J Am Assoc Lab Anim Sci 2011; 50: 471–478.
26 Mapletoft RJ, Bó GA. Superovulation in Cattle. In: Bovine Reproduction. John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015, pp 696–702.
27 Crowe M, Mullen M. Regulation and Function of Gonadotropins Throughout the Bovine Oestrous Cycle. 2013 doi:10.5772/53870.
28 Andino P. Evaluación de dos programas de superovulación en vacas lecheras. 2014.
29 Hesser M, Morris J, Gibbons J. Advances in recombinant gonadotropin production for use in bovine superovulation. Reprod Domest Anim 2011; 46: 933–942.
30 De Rensis F, Lopez-Gatius F. Use of equine chorionic gonadotropin to control reproduction of the dairy cow: a review. Reprod Domest Anim 2014; 49: 177–182.
31 Hesser MW, Morris JC, Gibbons JR. Advances in recombinant gonadotropin production for use in bovine superovulation. Reprod Domest Anim 2011; 46: 933–942.
32 Mapletoft RJ, Guerra AG, Dias FCF, Singh J, Adams GP. In vitro and in vivo embryo production in cattle superstimulated with FSH for 7 days. Anim Reprod 2015; 12: 383–388.
33 Mapletoft RJ, Bó GA. Superovulation in Cattle. In: Bovine Reproduction. John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014, pp 696–702.
34 Donaldson LE. LH and FSH profiles at superovulation and embryo production in the cow. Theriogenology 1985; 23: 441–447.
35 Ferr L, Bogliotti Y, Chitwood J, Kjelland M, Ross P. 234 hormonal follicle stimulation in holstein cows for in vitro embryo production using sperm sorted by flow cytometry. 2016 doi:10.1071/RDv28n2Ab234.
36 Kelly P, Duffy P, Roche J, Boland M. Superovulation in cattle: Effect of FSH type and method of administration on follicular growth, ovulatory response and endocrine patterns. 1997 doi:10.1016/S0378-4320(96)01589-8.
37 Carvalho PD, Hackbart KS, Bender RW, Baez GM, Dresch AR, Guenther JN et al. Use of a single injection of long-acting recombinant bovine FSH to superovulate Holstein heifers: a preliminary study. Theriogenology 2014; 82: 481–489.
38 Sharif MA, Kohram H, Zare Shahneh A, Zolfagharian H, Abedi Kiasari B, Hedayati M. Production and Purification of Equine Chorionic Gonadotropin Hormone Using Polyclonal Antibody. Iran J Biotechnol 2014; 12: 30–34.
39 Murphy B. Equine chorionic gonadotropin: an enigmatic but essential tool. Anim Reprod 2012; : 223–230.
40 Baruselli PS, Ferreira RM, Sales JN, Gimenes LU, Sa Filho MF, Martins CM et al. Timed embryo transfer programs for management of donor and recipient cattle. Theriogenology 2011; 76: 1583–1593.
41 Ararooti T, Niasari-Naslaji A, Razavi K, Panahi F. Comparing three superovulation protocols in dromedary camels: FSH, eCG-FSH and hMG. Iran J Vet Res 2017; 18: 249–252.
42 Boland MP, Goulding D, Roche JF. Alternative gonadotrophins for superovulation in cattle. Theriogenology 1991; 35: 5–17.
43 Añazco J, Vinueza N. Desarrollo de las estructuras ováricas en respuesta a la aplicación de dos gonadotropinas supeovulatorias en bovinos (PMSG y FSH-P). Carrera Ing. Agropecu. 2017.http://repositorio.espe.edu.ec/handle/21000/13423.
44 Capallejas RB, Rodríguez LT. Fisiología de la reproducción animal: con elementos de biotecnología. Editorial Félix Varela, 2009https://books.google.com.ec/books?id=iTuxAQAACAAJ.
45 Bó GA, Mapletoft RJ. Historical perspectives and recent research on superovulation in cattle. Theriogenology. 2014; 81. doi:10.1016/j.theriogenology.2013.09.020.
46 Genzebu D. A Review of Embryo Transfer Technology in Cattle. 2015.
47 Vongpralub T. Superstimulation of Follicular Growth in Thai Native Heifers by a Polyvinylpyrrolidone. 2013; 59: 4–8.
48 Callejas SS, Alberio R, Cabodevila JA, Dulout F, Aller J, Catalano R. El uso combinado de dosis reducidas de FSH-P y de eCG como tratamiento superovulatorio en bovinos *. Rev Argentina Prod Anim 2005; 276: 63–73.
49 Bó GA, Rogan DR, Mapletoft RJ. Pursuit of a method for single administration of pFSH for superstimulation in cattle: What we have learned. Theriogenology 2018; 112: 26–33.
50 Kimura K. Superovulation with a single administration of FSH in aluminum hydroxide gel: a novel superovulation method for cattle. J Reprod Dev 2016; 62: 423–429.
51 Ali M, Moustafa M Z. Effectiveness of a recombinant human follicle stimulating hormone on the ovarian follicles, peripheral progesterone, estradiol-17β, and pregnancy rate of dairy cows. Vet World 2016; 9: 699–704.
52 Looney C, Pryor J. Novel bovine embryo transfer technologies in the United States. Anim Reprod 2012; 9: 404–13.
53 Khan KH. Gene Expression in Mammalian Cells and its Applications. Adv Pharm Bull 2013; 3: 257–263.
54 Houdebine LM. Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis 2009; 32: 107–121.
55 Brinchmann MF, Patel DM, Pinto N, Iversen MH. Functional aspects of fish mucosal lectins—interaction with non-self. Molecules 2018; 23. doi:10.3390/molecules23051119.
56 Samaddar M, Catterall JF, Dighe RR. Expression of biologically active beta subunit of bovine follicle-stimulating hormone in the methylotrophic yeast Pichia pastoris. Protein Expr Purif 1997; 10: 345–355.
57 Min KS, Kang MH, Yoon JT, Jin HJ, Seong HH, Chang YM et al. Production of Biological Active Single Chain Bovine LH and FSH. Asian-Australas J Anim Sci 2003; 16: 498–503.
58 Wilson JM, Jones AL, Moore K, Looney CR, Bondioli KR. Superovulation of cattle with a recombinant-DNA bovine follicle stimulating hormone. Anim Reprod Sci 1993; 33: 71–82.
59 van de Wiel DF, van Rijn PA, Meloen RH, Moormann RJ. High-level expression of biologically active recombinant bovine follicle stimulating hormone in a baculovirus system. J Mol Endocrinol 1998; 20: 83–98.
60 Dirnberger D, Steinkellner H, Abdennebi L, Remy JJ, van de Wiel D. Secretion of biologically active glycoforms of bovine follicle stimulating hormone in plants. Eur J Biochem 2001; 268: 4570–4579.
61 Greenberg NM, Anderson JW, Hsueh AJ, Nishimori K, Reeves JJ, deAvila DM et al. Expression of biologically active heterodimeric bovine follicle-stimulating hormone in milk of transgenic mice. Proc Natl Acad Sci U S A 1991; 88: 8327–8331.
62 Coulibaly S, Besenfelder U, Miller I, Zinovieva N, Lassnig C, Kotler T et al. Expression and characterization of functional recombinant bovine follicle-stimulating hormone (boFSHalpha/beta) produced in the milk of transgenic rabbits. Mol Reprod Dev 2002; 63: 300–308.
63 Khodarovich YM, Goldman IL, Sadchikova ER, Georgiev PG. Expression of eukaryotic recombinant proteins and deriving them from the milk of transgenic animals. Appl Biochem Microbiol 2013; 49: 711–722.
64 Dyck MK, Lacroix D, Pothier F, Sirard MA. Making recombinant proteins in animals--different systems, different applications. Trends Biotechnol 2003; 21: 394–399.
65 Rudolph NS. Biopharmaceutical production in transgenic livestock. Trends Biotechnol 1999; 17: 367–374.
66 Wang Y, Zhao S, Bai L, Fan J, Liu E. Expression Systems and Species Used for Transgenic Animal Bioreactors. Biomed Res Int 2013; 2013: 9.
67 Toledo J, Ramos O, Montesino R, Fernandez Y, A Cremata J, Rodríguez Moltó MP. New procedure for production of biopharmaceutical proteins in the milk of non-transgenic animals. 2005.
68 Houdebine L-M. Preparation of Recombinant Proteins in Milk. Recomb Gene Expr; 267: 485–494.
69 Moura RR, Melo LM, Freitas VJ de F. Production of recombinant proteins in milk of transgenic and non-transgenic goats. Brazilian Arch Biol Technol 2011; 54: 927–938.
70 Khodarovich YM, Goldman IL, Sadchikova ER, Georgiev PG. Expression of Eukaryotic Recombinant Proteins and Deriving Them from the Milk of Transgenic Animals. 2013; 49: 711–722.
Received: 7 February 2019
Approved: 4 March 2019
Valeria M. Bautista Vega 1; Silvana P. Jiménez Chávez 2; Catherine D. Meza Franco 2; Thelvia I. Ramos 1; Jorge R. Toledo 2.
1 Departamento de Ciencias de la Vida y la Agricultura, Universidad de las Fuerzas Armadas – ESPE, Quito, Ecuador.
2 Biotechnology and Biopharmaceuticals Laboratory, Department of Physiopathology; School of Biological Sciences. Universidad de Concepción. Victor Lamas 1290, P.O. Box 160C, Concepción, Chile.
