2021.06.03.4
Files > Volume 6 > Vol 6 No 3 2021
INVESTIGATION / RESEARCH
Assessing HeberFast® Line Gavac, a lateral flow immunochromatographic system for the rapid detection of anti-Bm86 antibodies in Gavac vaccinated cattle
Milagros Vargas-Hernández*1, Yeni Hernández Lorenzo2, Viviana Pluma Perez2, Isabel Rosales-Garcia2, Sunamit Rodríguez-Mendez2, Enrique Pérez-Cruz3, Daymi Abreu-Remedios3, Carlos Montero-Espinosa1, Ayme Oliva-Cardenas1, Elaine Santana-Rodriguez1, Danny Pérez-Pérez1, Yusmel Sordo-Puga1, Yohandy Fuentes-Rodríguez1, Alianne Fundora-Llera1, Carlos A. Duarte1, Ernesto Galbán-Rodríguez4, Carlos Hernandez-Diaz3, Dayamí Dorta Hernandez3, Ivis Pasaron Rodriguez3, Marisela Suarez-Pedroso1
Available from: http://dx.doi.org/10.21931/RB/2021.06.03.4
ABSTRACT
Rhipicephalus Boophilus microplus cattle tick is a scourge for livestock production. The infestations produced by this pathogen are incompletely contained by chemical treatments, with the associated environmental pollution risks. Vaccination against cattle ticks has emerged as a feasible and environmentally friendly strategy to control tick-borne diseases. In this setting, Gavac® vaccine has proven effective in decreasing cattle tick populations through antibody responses against the tick Bm86 antigen, as part of an Integrated Control Program. However, animal vaccination programs require easy and ready-to-use screening tests to follow up the immune response in vaccinated animals under field conditions. This study reports the evaluation HeberFast® Line Gavac, a lateral flow immunochromatographic system for the rapid detection of anti Bm86 antibodies in vaccinated cattle. The system was tested on 598 serum samples taken from immunized animals, arranged in three groups according to their anti-Bm86 antibody response in ELISA (209 high, 150 medium or 239 low and 100 samples from non-immunized animals. The HeberFast® Line Gavac system was assessed for sensitivity, specificity, and concordance against the ELISA reference test. Consistency was evaluated among production batches and inter-analyst reading-independent consistency at two moments: ten minutes after completing the test and after strip drying. The system showed high sensitivity (81.6%, 82.2%, and 81%), specificity (96.7, 94.6, and 93.3%), and agreement with the ELISA reference test (75%; 74%, and 71%) for high, medium and low anti-Bm86 sera, respectively. The effectiveness of the diagnosis was 87.6; 87.1; 85.9 for high, medium, and low antibody titers, respectively. Consistency among production batches and analysts was documented, and no significant differences between evaluation times were found. These results indicate that HeberFast® Line Gavac is a valuable tool for the serological surveillance of Gavac vaccinated cattle.
Keywords: Cattle tick, vaccination, antibody screening strip, lateral flow immunoassay, Bm86, Gavac®.
INTRODUCTION
Rhipicephalus (Boophilus) microplus cattle tick is a scourge for livestock production in tropical and subtropical areas. Ticks cause significant losses due to their direct weakening effects in cattle and through tick-borne diseases, ultimately causing animal death1. In Cuba, an Integrated Management Program for cattle tick control has been implemented nationwide. This program comprises the combination of Gavac®, a recombinant vaccine against cattle tick, with chemical acaricide treatments, scheduled according to the infestation index, and other measures for the biological, mechanical, immunological, and genetic control of the plague. The application of the vaccine has effectively sparse the number of acaricide treatments required, thereby reducing the environmental pollution risks and diminishing a 98% the deaths rates caused by hemoparasites2-4.
The active ingredient of Gavac® is the tick gut Bm86 protein. Anti-Bm86 antibodies induced upon vaccination will attach to the gut epithelium of suckling ticks, with deleterious effects on their populations and the size of their progeny5,6. Regular immunizations are required to maintain protective anti-Bm86 antibody titers in the blood of the animals6-8.
Commonly, antibody titers in sera of immunized animals are monitored through antigen-specific Enzyme-Linked Immunosorbent Assays (ELISA) tests, high efficiency, and specificity. However, such assays are troublesome under field conditions, time-consuming, and require specialized laboratory facilities and qualified personnel to perform and evaluate the results objectively. Thus, a fast and easy-to-use screening test is needed to implement and evaluate ongoing, and future cattle tick vaccination campaigns under field conditions.
Therefore, this study aimed to evaluate the performance of HeberFast® Line Gavac, a fast chromatographic screening test for the detection of anti-Bm86 antibodies, and its concordance with the reference ELISA test used in the laboratory. The main parameters to compare were sensitivity, specificity, and consistency.
MATERIAL AND METHODS
Material and Reagents
Ethical statement
Blood was extracted from cattle following an experimental protocol elaborated according to the ethics guidelines for animal care 9 and approved by the Ethics Committee of the Center for Genetic Engineering and Biotechnology (CIGB).
Serum collection and measurement of anti-Bm86 antibody titers by ELISA
Positive and negative anti-Bm86 antibody blood was collected from animals in units either under tick control programs with Gavac® vaccination or without vaccination. Blood was taken from healthy cattle, regardless of age, sex, or reproductive categories, and the sera were stored at –20oC until use.
ELISA measured the level of anti-Bm86 antibodies in sera. Briefly, ELISA microplates (Polysorp, Nunc, USA) were coated with Bm86 protein at 2 µg/mL and incubated overnight at 4ºC. Plates were washed with PBS-Tween 20 (0.05%), and blocked with 1% skimmed milk for 1 h at 37ºC. Sera was next diluted 1:500 in PBS-Tween 20 (0.05%) and 100 μL added to each well, and further incubated for 1 h at 37ºC. Next, a rabbit anti-bovine IgG antibody conjugated to horseradish peroxidase was added for 30 min at 37ºC. Finally, the reaction was developed with 0.5 mg/mL ortho-phenylenediamine and 0.015 % H2O2, and the reaction stopped 10 min later with H2SO4 (1%). The optical density (OD) was measured at 492 nm in an ELISA plate reader (Sunrise, Austria). A standard curve made with five 1:2 serial dilutions of an anti Bm86 positive reference serum reference, starting with 1:640, was used to interpolate the OD values of each serum. The anti Bm86 antibody values were expressed as absorbance units (AU)/mL. Three groups were formed according to the results of this evaluation:
- Group 1. High responders: 209 positive samples with anti-Bm86 antibody values higher than 1000 AU/mL.
- Group 2. Medium responders (gray zone): 150 samples with anti-Bm86 antibody values between 530 and 1000 AU/mL (minimum protective titers).
- Group 3. Low responders: 239 samples with non-protective (negative) antibody values lower than 530 AU/mL.
- Another group of negative sera with 100 samples from non-immunized animals was conformed.
HeberFast® Line Gavac
Bm86 protein and its conjugation to colloidal gold
The active pharmaceutical ingredient in GAVAC® vaccine was supplied by its manufacturer, the Center for Genetic Engineering and Biotechnology of Camagüey, Cuba. The conjugation of the Bm86 protein to colloidal gold was carried out according to Bailes et al., 2012,10 using homemade 20 nm colloidal gold particles. The protein was solubilized in 0.005 mol/L of sodium chloride. The colloidal gold was also adjusted to pH 7 with 0.1 M K2CO3.
The following flocculation test first determined the amount of protein in relation to a fixed volume of gold: 500 µL of 20 nm colloidal gold at pH 7 were mixed with 100 µL of the protein solution in a range between 4 and 20 µg. The mixture was homogenized and incubated for 10 min at room temperature, and 500 µL of 10% sodium chloride solution was added to each reaction mixture. Finally, the conjugate content in the mixture was quantified by measuring the absorbance at 530 nm in a spectrophotometer (Thermo Scientific GENESYS 10S UV-Vis, Madison, USA).
Once the optimal amount of protein had been selected, the reaction was conducted for 30 min at -20 °C - 25 °C, stirring at 300 rpm. The free sites on the colloidal gold particles were blocked with an adequate volume of 10% BSA, for a final concentration of 0.5 %. The final mixture was centrifuged at 16000 x g for 1 hour at 4⁰C. The conjugate obtained (pellet) was suspended in 0.1 M TBS, BSA 1% pH 7.0, and diluted up to an absorbance of 40 at a wavelength of 530 nm.
Preparation of the immunoreactive strip
The strip components were nitrocellulose (NC) membrane of 15 µm (MDI, type CNPC-SS12-L2-H50, dimensions 7,5 x 26 cm, 1,5 cm of NC), pad for the antigen-gold conjugate (MDI, type PTR-5, dimensions 2,7 x 26 cm), and absorbent pad (MDI, type AP080, dimensions 3,5 x 26 cm) . The conjugate was diluted to an absorbance of 20, at a wavelength of 530 nm and dispensed onto the antigen-gold conjugate pad.
The Bm86 protein, dissolved at 4 mg/mL in phosphate buffer saline (PBS) was absorbed into the membrane in the test line. The mixture of the control line components consisted of a monoclonal antibody against Bm86 protein provided by the Center for Genetic Engineering and Biotechnology of Sancti Spíritus, Cuba at 4 mg/ml, and 0.2 mg/mL of poly L-lysine, dissolved in PBS.
Both the macroporous material and the nitrocellulose membrane were sprayed with the BioDotQuanti 2000, BioJet, England, and then dried in an oven at 37 °C, for 30 min. The NC membrane cards were blocked for 1h at room temperature in blocking solution (0.02% (v/v) Tween 20, 0.02% (w/v) sodium azide, 5% (w/v) sucrose, and 0.5% bovine seroalbumin (BSA), dissolved in PBS. Later they were dried in the oven at 37 °C for 1 h.
Test strip assembly
The NC membrane was coupled in the center of polyvinyl chloride support. The absorbent pad and the conjugate pad were glued by overlapping 1 mm at the top and bottom of the NC Membrane, respectively. The sample pad was then glued by overlapping 2 mm on the bottom of the conjugate pad. The master assembled card was cut into 4 mm wide strips using a CM5000-guillotine-cutter (BioDot, Irvine, USA).
Immunochromatographic assay
The rapid, one-step, and visual reading lateral flow immunoassay (LFIA), branded HeberFast® Line Gavac, consists of a strip of nitrocellulose (NC) membranes to detect antibodies against the Bm86 antigen in bovine serum. The membrane consists of two phases: a fixed phase composed of the Bm86 protein as a capture biomolecule for the specific immunoglobulins present in the serum and a mobile phase containing a protein-colloidal gold marker conjugate, which finally develops the reaction with a specific dye.
Each strip was introduced into 80 µL of bovine serum (previously diluted 1:8 in buffer). The diluted sera migrated by capillarity within the macroporous material, dissolving the Bm86 conjugate labeled with colloidal gold. Two independent reactions could be observed in the virtual window. If the sample was positive, the immune complex reacted with the Bm86 protein attached to the NC membrane, forming a first horizontal redline Positive Line (PL). For all samples, the free conjugate was captured in a second red horizontal line Control Line (CL) where a polyclonal anti-IgG murine antibody is fixed to the solid phase.
Performance evaluation
Strips were used to detect antibody responses in 698 samples of bovine sera, from598 vaccinated and 100 unvaccinated animals. The results of the samples were interpreted at two moments, at the end of the reaction 10 min after the sample was applied and once the strip dried. LFIA tested samples in triplicate. The specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), efficacy as well as kappa coefficient (K), were determined.
Kappa coefficient (K)
The Kappa coefficient was calculated to assess the level of significance of the agreement between the ELISA and the LFIA. The following ranges and their respective performance criteria were defined: 0.81-1.00, almost perfect agreement; 0.61-0.80, reasonable agreement, 0.60-0.41, moderate agreement; 0.40-0.21, discrete agreement, 0.20-0.01 insignificant agreement, and lower than 0.01, no agreement11.
Batch to batch consistency
The samples from all panels were all evaluated by the three batches of strips (Gavac1501, Gavac1502, and Gavac1503). Results were considered valid for samples with coincident results with two different strip batches.
Inter-analyst interpretation
Three analysts read each sample, and results were regarded as valid when two out of the three observers coincided.
Statistical analysis
The LFIA and the ELISA reference system results were statistically compared using the epidemiological analysis of tabulated data (EPIDAT) software, version 3.112. Concordance, sensitivity, specificity, efficacy, positive and negative predictive values, batch to batch consistency, and inter-analyst interpretation were compared. Statistical significance was considered for a 95 % confidence interval.
RESULTS
Evaluation of the panel of samples from unimmunized animals
All sera from unimmunized animals were negative. Only one signal appeared in the diagnostic control line, which guarantees the validity of the test. This represented a 100% of specificity for the system. Four representative samples from this group are shown in figure 1.

Figure 1. Evaluation of sera from Gavac immunizes animals and unvaccinated controls. Four representative samples per group were included. A: unvaccinated control group; B: high responders, (>1000 AU/mL); C: medium responders (530 - 1000 AU/mL); D: low responders (>530 AU/mL).
Evaluation of samples from immunized animals
All the strips used were accepted as valid, as the control line indicating their quality and reliability was observed. The intensity of the test capture region line varied depending on the concentration of immunoglobulins in the sample.
The overall false positive rate fluctuated between 3.34% and 6.69%, while a range of 4.78%-5.26% was found for false-negative results for the different lots and times evaluated. As expected, the percentage of false negative and false positive values was highly dependent on anti-Bm86 antibodies in the samples. Samples from group 2 (medium responders) showed the greatest incongruities when compared to the reference ELISA (36% - 38.7% of false negatives) (Table 1). The percentage of false-negative values dropped to 4.8%-5.2% in the group of high responders. On the other hand, the percentage of false positives in the ELISA negative sera was only 3.4-7.1%. Figure 1 shows 4 representative samples from each of the three groups evaluated.

Table 1. Evaluation of the diagnostic strips with a panel of positive anti-Bm86 sera
The general agreement of the immunochromatographic strips with respect to ELISA was good for the 3 batches evaluated based on the kappa coefficient (Gav1501, Gav1502, and Gav1503) (Table 2).

Table 2. General agreement between the immunochromatographic assay and the reference ELISA
The results of sensitivity, specificity, efficacy (test validity index), positive and negative predictive values, and confidence intervals of the 3 batches in studies are shown in Table 3.

Table 3. Performance of the immunochromatographic system
Consistency between batches
The agreement among the three batches of strips evaluated (Gav1501, Gav1502, and Gav1503) is shown in Table 4. All batches exhibited high kappa coefficients, which indicate a good, or almost perfect degree of concordance. These results demonstrated the consistency of the manufacturing process.
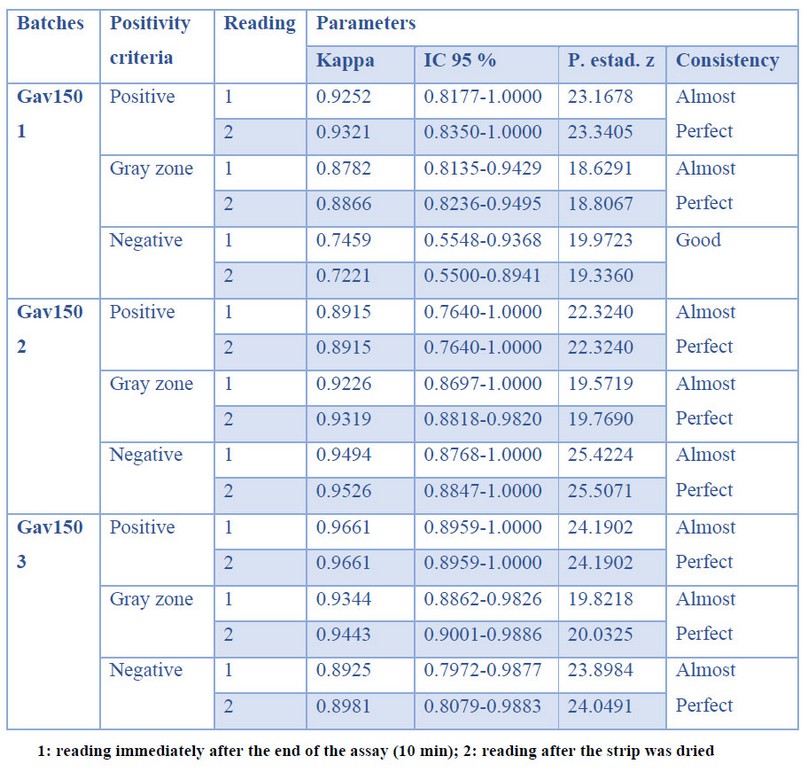
Table 4. Consistency in production
Independence of the analysts
The concordance among the three analysts that read the assays ranged from good to almost perfect as the concentration of immunoglobulins increased. The results are shown in Table 5. The samples evaluated in the two moments gave no significant differences.
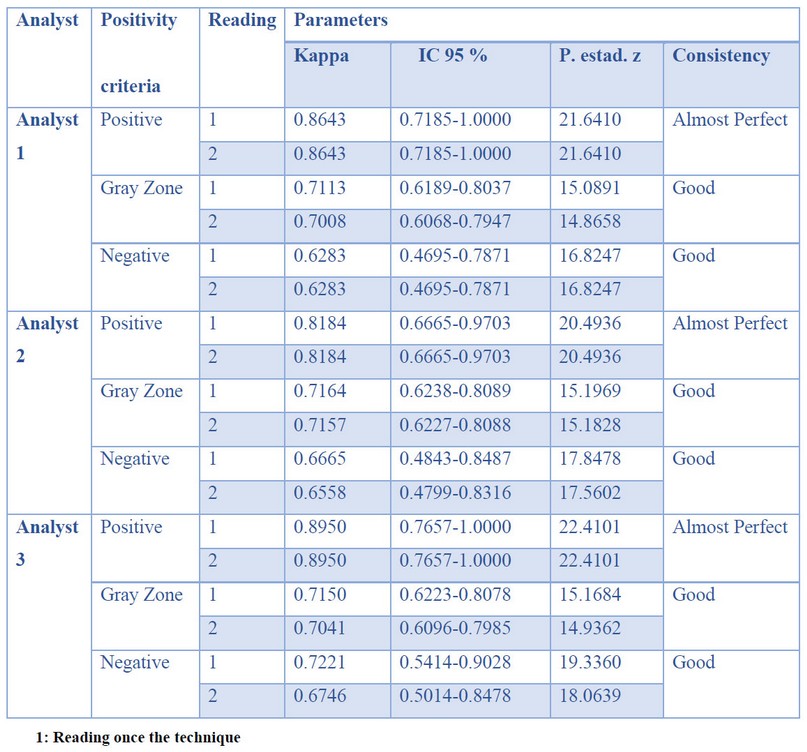
Table 5. Analyst Interpretation
DISCUSSION
Tick mitigation strategies are part of a long tradition and focus on the local application of acaricides or the search for biological products that reduce the impact of the problem13. The use of the Gavac® immunogen within a control program decreases the reproductive parameters of the tick through the specific antibodies developed in the immunized animals14. Moreover, the fast development of anti-Bm86 antibody levels guarantees herds immunity and the effective control of tick populations4.
In this setting, the development of rapid, specific, sensitive, and economically feasible diagnostic methods such as LFIA is of paramount relevance in veterinary medicine. They support the massive screening of samples in a shorter time under field conditions and the early detection of pathogens, further providing specific treatments and monitoring for the control epidemiological units15.
In this study, the performance of the HeberFast® Line Gavac immune screening test was evaluated for detecting anti-Bm86 antibody titers in sera of immunized cattle. The sensitivity and specificity of the immunochromatographic system for the three batches under study were in the 81-82% and 92-95% ranges, respectively. Similar results have been previously reported for the use of LFIA strips for massive screening. For instance, the direct Sensitive Membrane Antigen Rapid Test (SMART®; New Horizons Diagnostics Corp., USA) and the direct Pathogen Detection Kit® (PDK; Intelligent Monitoring Systems, USA) were reported to display 100 % sensitivity for cholera diagnosis in diarrheal and semi-formed stool samples, and in intestinal contents of corpses. Furthermore, 100 % specificity was also attained with SMART®, while the same parameter for direct PDK® ranged between 77.4% and 85.7%, depending on sample type16. In immunochromatographic studies to detect Giardia spp. and Cryptosporidium spp. in feces, 58-97.2 % sensitivity and 99-100 % specificity were reported 17,18.
In summary, diagnostic tests must perform with high specificity and sensitivity to avoid false positives or false negative results. This is more relevant when diagnosing severe illnesses or other conditions able to compromise herd health and integrity and devoid of any contingency measures other than animal sacrifice. A misdiagnosis can lead to serious economic losses in those situations, as forum treatable pests such as Foot and Mouth Disease, African Swine Fever, Classical Swine Fever, and similar 19,20.
Since the HeberFast® Line Gavac strip will be applied to determine the immune status of animals vaccinated with Gavac®, the specificity of this test is more critical than its sensitivity. Consequently, the essay must exhibit a low fraction of false positives when assessing post-vaccination immunity. The assay's specificity was in the range 92.9-95.4% for the three batches tested, well above its sensitivity values (81-82.4%).
In veterinary medicine, lateral flow immunoassays have been developed for various purposes; detection of diseases in companion and farm animals to achieve better results in the care and development of animals, for example, Parvovirus21, Newcastle22, Avian infectious bronchitis virus23, African swine fever24and Babesiosis25, The rapid detection of these diseases reduces the economic losses. Immunochromatographic methods have also been developed to detect antibiotics and analogs in milk26, which is greatly important for food safety.
In general, this test offers advantages and benefits, especially in the field, due to its fast and safe use and relatively low production costs. Moreover, it does not require equipment for readouts, and the results are easily interpreted with the naked eye, with high specificity and sensitivity in just 10 min. Finally, this immunoscreening test has been developed to work with serum, a sample that can be easily collected and processed.
HeberFast® Line Gavac is the first rapid kit worldwide for the detection of antibodies against the Bm86 protein. Its introduction in the field could facilitate the follow-up of the immune response against any vaccine based on this antigen and determine the level of immunity achieved in the vaccinated herds.
CONCLUSIONS
The HeberFast® Line Gavac immunochromatographic system allows the determination of the anti-Bm86 serological status quickly and straightforwardly to a large number of samples in field conditions with minimal effort. The introduction of this test would significantly improve the follow-up of the antibody response to GAVAC in all the country as part of the Integrated Control Program for cattle tick control.
Acknowledgements
This work was carried out thanks to the collaboration with the Unit of Central Laboratories of Agricultural Health (ULCSA), Ministry of Agriculture, Cuba
REFERENCES
1. Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitology research 2018; 117:3-29.
2. Valle MR, Mendez L, Valdez M, et al. Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine Gavac. Experimental & applied acarology 2004; 34:375-82.
3. Suarez PM, Méndez, M.L., Valdez, M., Souza, R.M., Camargo, A.J.R., Constanza, N.V., Ascanio, E.E. Control de las infestaciones de la garrapata Boophilus microplus en la ganadería Cubana y en regiones de latinoamérica con la aplicación del inmunógeno Gavac® dentro de un programa de lucha integral. Documento sexta conferencia Electrónica - Redectopar 2007.
4. Suarez M, Rubi J, Pérez D, et al. High impact and effectiveness of Gavac™ vaccine in the national program for control of bovine ticks Rhipicephalus microplus in Venezuela. Livestock Science 2016; 187:48-52.
5. Willadsen P, Kemp D. Vaccination with ‘concealed’antigens for tick control. Parasitology Today 1988; 4:196-8.
6. de la Fuente J, Rodriguez M, Redondo M, et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine 1998;16:366-73.
7. Lamberti J, Signorini A, Mattos C, et al. Evaluation of the recombinant vaccine against Boophilus microplus in grazing cattle in Argentina. Recombinant Vaccines for the Control of the Cattle Tick (ed De la Fuente, J) 1995:205-27.
8. Vargas M, Montero C, Sanchez D, et al. Two initial vaccinations with the Bm86-based Gavacplus vaccine against Rhipicephalus (Boophilus) microplus induce similar reproductive suppression to three initial vaccinations under production conditions. BMC veterinary research 2010;6:43.
9. Ethical Guidelines for the Use of Animals in Research. 2018. (Accessed 23/10/2021, 2020,
10. Bailes J, Mayoss S, Teale P, Soloviev M. Gold nanoparticle antibody conjugates for use in competitive lateral flow assays. Nanoparticles in Biology and Medicine: Springer; 2012:45-55.
11. Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics 1977:159-74.
12. EPIDAT 3.1. Una herramienta para el análisis epidemiológico de datos tabulados. Infomed, 2005. (Accessed 14//05/2020, 2020, at http://www.sld.cu/sitios/revsalud/temas.php?idv=1178.)
13. Las garrapatas del bovino y los agentes de enfermedad que transmiten en escenarios epidemiológicos de cambio climático. Guía para el manejo de garrapatas y adaptación al cambio climático. Instituto Interamericano de Cooperación para la Agricultura (IICA), 2016. (Accessed 12/06/2020, 2020, at http://creativecommons.org/licenses/by-sa/.0/igo/.)
14. Vargas-Hernández M, Santana-Rodríguez E, Sordo-Puga Y, et al. Stability, safety and protective immunity of Gavac® vaccine subjected to heat stress. Biotecnología Aplicada 2018; 35:1221-7.
15. Sastre P, Gallardo C, Monedero A, et al. Development of a novel lateral flow assay for detection of African swine fever in blood. BMC veterinary research 2016; 12:1-8.
16. Bolaños HM, Acuña MT, Serrano AM, et al. Desempeño de los sistemas Cholera-SMART® y Pathogen-Detection-Kit® en el diagnóstico rápido del cólera. Revista Panamericana de Salud Pública 2004; 16:233-41.
17. Corripio IF, Cisneros MJG, Ormaechea TG. Diagnóstico de las parasitosis intestinales mediante detección de coproantígenos. Enfermedades Infecciosas y Microbiología Clínica 2010; 28:33-9.
18. Gutiérrez-Cisneros MJ, Martínez-Ruiz R, Subirats M, Merino FJ, Millán R, Fuentes I. Evaluación de dos métodos inmunocromatográficos comerciales para el diagnóstico rápido de Giardia duodenalis y Cryptosporidium spp. en muestras de heces. Enfermedades Infecciosas y Microbiología Clínica 2011; 29:201-3.
19. Knowles N, Samuel A. Molecular epidemiology of foot-and-mouth disease virus. Virus research 2003; 91:65-80.
20. OIE. Chapter 15.2. Infection with classical Swine fever virus. Terrestrial Animal Health code. In: OIE, ed.2019.
21. Sharma C, Singh M, Upmanyu V, et al. development and evaluation of a gold nanoparticle-based immunochromatographic strip test for the detection of canine parvovirus. Archives of virology 2018; 163:2359-68.
22. Li Q, Wang L, Sun Y, et al. Evaluation of an immunochromatographic strip for detection of avian avulavirus 1 (Newcastle disease virus). Journal of Veterinary Diagnostic Investigation 2019; 31:475-80.
23. Liu I-L, Lin Y-C, Lin Y-C, Jian C-Z, Cheng I-C, Chen H-W. A novel immunochromatographic strip for antigen detection of avian infectious bronchitis virus. International journal of molecular sciences 2019; 20:2216.
24. Wang X, Ji P, Fan H, et al. CRISPR/Cas12a technology combined with immunochromatographic strips for portable detection of African swine fever virus. Communications biology 2020; 3:1-8.
25. Stuart Tayebwa D, Magdy Beshbishy A, Batiha GE-S, et al. Assessing the immunochromatographic test strip for serological detection of bovine babesiosis in Uganda. Microorganisms 2020; 8:1110.
26. Peng J, Liu L, Kuang H, Cui G, Xu C. Development of an icELISA and immunochromatographic strip to detect norfloxacin and its analogs in milk. Food and Agricultural Immunology 2017; 28:288-98.
Received: 02 February 2021
Accepted: 02 June 2021
Milagros Vargas-Hernández*1, Yeni Hernández Lorenzo2, Viviana Pluma Perez2, Isabel Rosales-Garcia2, Sunamit Rodríguez-Mendez2, Enrique Pérez-Cruz3, Daymi Abreu-Remedios3, Carlos Montero-Espinosa1, Ayme Oliva-Cardenas1, Elaine Santana-Rodriguez1, Danny Pérez-Pérez1, Yusmel Sordo-Puga1, Yohandy Fuentes-Rodríguez1, Alianne Fundora-Llera1, Carlos A. Duarte1, Ernesto Galbán-Rodríguez4, Carlos Hernandez-Diaz3, Dayamí Dorta Hernandez3, Ivis Pasaron Rodriguez3, Marisela Suarez-Pedroso1
1Departamento de Biotecnología Animal, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología, CIGB.Ave. 31 e/ 158 y 190, Cubanacán, Playa, La Habana, CP 11600, Cuba.
2Unidad de Laboratorios Centrales de Sanidad Agropecuaria (ULCSA), Ministry of Agriculture, Cuba.
3Centro de Ingeniería Genética y Biotecnología, Santi Spirítus, Cuba.
4Editorial Elfos Scientiae, Dirección de Promoción y Distribución Nacional, CIGB
Corresponding author: Milagros Vargas Hernández. Centro de Ingeniería Genética y Biotecnología, Apdo6162, Playa, La Habana 10600, Cuba. Phone: 53-7-2504421, Fax: (53-7) 271 4764. Email: [email protected]
