2022.07.02.8
Files > Volume 7 > Vol 7 No 2 2022
Probe on Various Experimental Cigarette Smoke Subjection Structure
Moulima Das 1*, Anupam Saha 1
1 M.Pharm Grad., Pharmacology, NSHM College Of Pharmaceutical Technology, NSHM Knowledge Campus, B.L. Rd., Kolkata - 700053, WB India; [email protected]
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.8
ABSTRACT
Different methods of subjection to smoke from experimental cigarettes are essential for understanding tobacco smoke. The major toxicants found in tobacco are acetaldehyde, acetone, acrolein, acrylonitrile, ammonia, benzene, cadmium, catechol, chromium, cyanide hydrogen, arsenic, nickel, nitric oxide, nicotine last but not least, mono-oxide gases. While experts say, cigarette smoke contains more than 4000 different compounds. These are substantially toxic and can destroy cells, and many of them are carcinogenic. Various smoke-exposure devices are used for in-vitro tobacco smoke generation, dilution, and distribution.
Such devices are used widely by well-known manufacturers or can be tailor-made setups. We can set up different in-vitro models to better treat smoke-related diseases using these subjection structures. The fundamental goal will be to build a tobacco-free society of available subjection systems. Some have been identified and established as biological endpoints in some published scientific literature. In the scientific field, many new technologies are coming out and showing their presence. There are many systems of exposure to cigarette smoke in vitro which offer a more flexible approach to the challenges of exposure to tobacco smoke. This review covers some topics such as the description of available new subjection structures and reviews their work, setting up and application for Scenarios of in-vitro treatment. The benefits and disadvantages of both subjection mechanisms and the similarities between the setups and the data extracted from these structures. Measuring the smoke dose is also discussed here as an important field of research, particularly in the preclinical phase.
Keywords: Cigarette smoke; Cigarette Subjection Structures; Cigarette Subjection Mechanisms; Cigarette Subjection Advantages; Cigarette Subjection Use; Cigarette Subjection Modern advancements.
INTRODUCTION
Most findings of respiratory disease relate specifically to tobacco consumption. Comprehension of the complex dynamics of tobacco smoke is fundamental, which can allow the precursors and mechanisms responsible for adverse health effects 1. The smoke from tobacco kills up to half its users. More than 8 million people are killed by cigarettes each year. About 7 million of those deaths result from direct use of cigarettes, while about 1.2 million results from second-hand smoke exposure by non-smokers 1,4,6. Tobacco causes severe injuries to the health because of hazardous chemical compounds such as nicotine, cadmium, lead, polonium-210, benzene, acrylonitrile, various aldehydes, aromatic amines, aromatic polycyclic hydrocarbons 2,6. The compositions are related to COPD, lung toxicity, and multiple cancers.
In-vitro tobacco smoke measurement is conducted on the particulate process collected on a Cambridge filter pad and eluted in DMSO or bubbled through the media or PBS to cell culture 3. The cell cultures are then exposed to the particulate phase under submerged conditions. Sadly, particulate-based exposures do not consider the period of cigarette smoke vapor or the related interactions between the level of particulate matter and vapor 3,4. Aquatic agricultural environments and particulate-based concentrations do not match the human lung's primary stream exposure to tobacco smoke. Separating fractions of smoke in this manner could also contribute to alterations 5. The chemical changes may not indicate the smoke aerosol as a whole. Whole smoke exposure systems were developed to overcome the problems 6. Different whole-smoke exposure systems are available worldwide, and most of them in Germany is very popular. There must be some drawbacks, but they could play an important role and bridge the gap between technologies, not only in calculating the actual cell dosage but also in characterizing and validating these systems 5,7. A review article covers recent advances in various subjection systems for cigarettes, their mechanisms of action and their application in different fields 3,7. These are very useful for understanding the processes of the cell damage and disease mechanisms caused by tobacco 8. This review describing tobacco smoke and disease, elucidating disease processes and defining smoke toxicants responsible for adverse health effects will be critical in-vitro approaches using dose instruments will add strength to the in-vitro data resulting from this 9.
Whole Smoke Exposure Systems
Traditional techniques of exposure to smoke are based on the particulate phase of cigarette smoke and omit any subsequent analysis of the vapor and semi-volatile phase. Whole smoke exposure systems allow evaluating all stages of smoke together or separately, depending on the experimental setups 5,7,10. This has enabled researchers to conduct their experiments to investigate tobacco smoke phases, yielding valuable information. Some of the systems on the market include Bespoke 11, Borgwaldt 12, Burghart 13, Vitrocell 14, and Cultex 15. Among these is the more advanced Vitrocell system. A summary of different whole smoke exposure systems can be found in table 1.
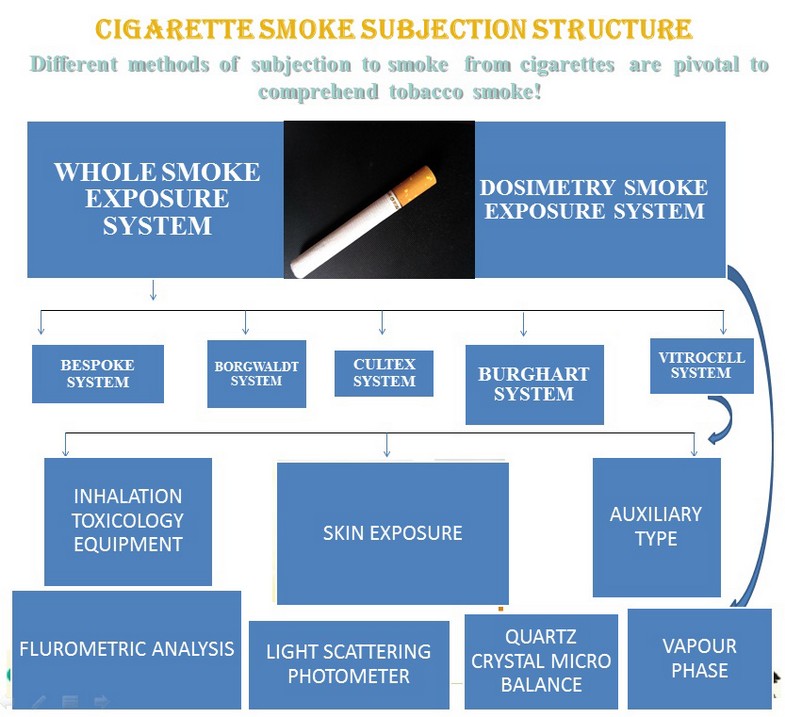
Figure 1. Graphical abstract on various cigarette smoke subjection structures.

Table 1. Comparison between different types of whole smoke subjection structures.
Vitrocell Systems
A brief detailing with advancements of Vitrocell systems where now a days this is the advanced in-vitro exposure systems. The system's goal is to assist in attaining the research goal by working hand in hand with the researchers. This integral commitment to one’s research means one can count on the system to propose optimally tailored components 40, provide the latest installations 41, an international training and a world-class service precisely to the requirements of the researchers.
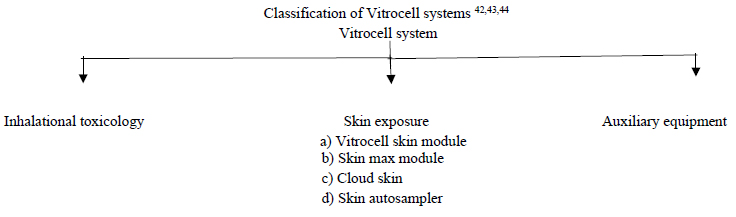
Vitrocell Inhalational Toxicology 45,46,47
They advanced device systems for in vitro characterization of airborne substances at the air / liquid interface, such as gases, complex mixtures, nanoparticles and fibers. The device design considers the type of aerosol, the insert sizes of the cell culture, the mammalian cell system and the dosimetry. The details of this type are illustrated in table 2, table 3 and table 4, respectively.

Table 2. Details of vitrocell inhalational toxicology system.

Table 3. Some features of the aerosol generator systems used in Vitrocell systems 48,49.
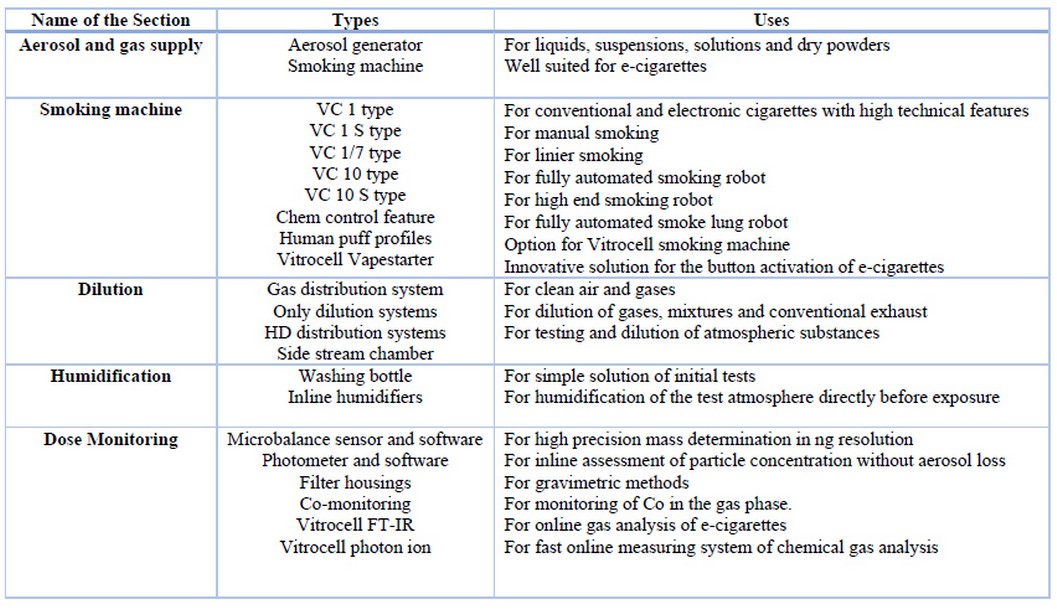
Table 4. Different parts and their uses of Vitrocell Inhalational Toxicology 50,51,52.
Vitrocell Skin Exposure Systems 53,54,55
High tech equipment solutions for the exposure of skin tissue to solid, liquid or gaseous substances. VITROCELL Skin modules are a powerful alternative to glass-made Franz cells as they offer improved handling, higher throughput and options for automation.

Table 5. The various types, their parts and some essential features.
Vitrocell Auxiliary Equipment 56
A range of durable components for a successful and long-lasting operation of our exposure systems. Own equipment solutions were developed when commercial solutions are not available. VITROCELL approved auxiliary equipment fits perfectly into our turnkey installations.
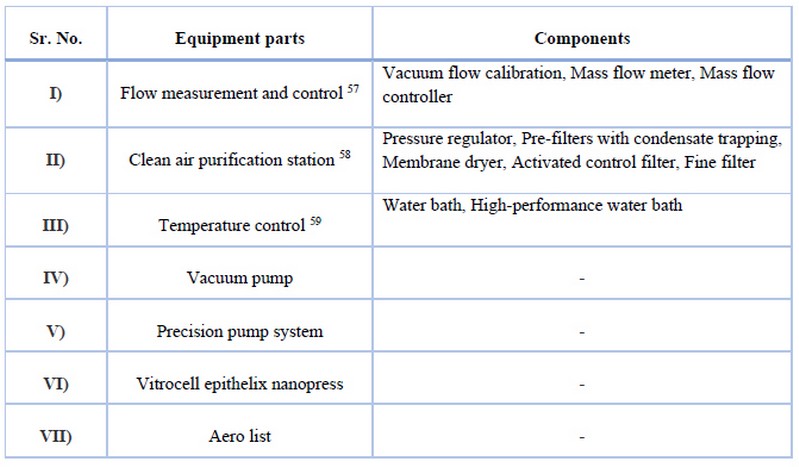
Table 6. The details of the equipment are given below.
Dosimetry for smoke exposure systems
This system is essential for understanding exactly which components of smoke are exposed to the culture of cells. Tobacco smoke has two phases that can cause injury to the lung and damage to cells 60. The dosimetry system is thus helpful in understanding the characteristics and interactions of both steps 61. The different tools are used for particle deposition assessment under dosimetry are-fluorometric analysis 62, light scattering photometers 63, quartz crystal microbalance 64, and vapor phase 65.

Table 7. Several dosage tools available for particulates and vapors are explored in more detail.

Figure 2. (a) Fluorometric analysis (Wet chemistry technique): A susceptible instrument, the wet-chemistry-based fluorimeter, is a reproducible instrument used for all fluorometric applications. The key advantages are precision and versatility 64—four fluorescent channels in common nucleic acids and protein assays. Fluorimeters based on wet chemistry can ultimately personalize the measuring applications 66. This instrument has powerful software and full network integration to handle the data seamlessly. This figure is obtained from MRC Laboratory-Instruments, (Available: https://www.mrclab.com/portable-fluorometer-uv-blue-channels); (b) Light scattering photometers: Light scattering photometer is essential to determine absolute molar masses from cigarette ignition. (Molar mass range-200 to million g/mole and 10nm to 50nm roots means square radii). It is an excellent method for characterizing polymer and protein in different inquiries, or product quality controls 67. This figure is obtained from Holmarc Opto-Mechatronics PVT Ltd, Available: https://www.holmarc.com/scattering_photometer_-_single_channel_light_detection.php).
Recent findings on different issues of the whole smoke exposure system:-
These subjection structures may be too unique in their evolution to be widely used for a consistent approach. No commercially available or otherwise available exposure system has yet been fully characterized or validated, and each system offers unique advantages and inconveniences 17. Interestingly, an assessment by the Cooperation Authority for Scientific Research Relative to Tobacco (CORESTA) on various whole smoke exposure technologies – a tobacco-related task force in vitro, found remarkably consistent results, indicating the similar performance of these systems (CORESTA air-liquid interface report). Entire smoke exposure systems are an important development for producing an in vitro physiologically relevant test smoke aerosol 18. In support of this, the Committee on Mutagenicity in the UK reviewed the field of 'chemicals in food, consumer goods, and the environment' in June 2009 and commented that the implementation of whole-smoke exposure protocols was likely to provide more specific data on tobacco smoke's mutagenic effect, but noted that none of the test systems had been 'successfully validated'32. Validation remains a prominent area for improvement in this research field, and no one has yet carried out a multi-laboratory or multi-system study. Several models, techniques, findings, limitations, and further suggestions are explored in more detail in Table 8. Quartz crystal microbalance takes advantage of the piezoelectric character of quartz to characterize the formation and structural properties of thin films in real-time. This is done by simultaneously measuring the changes in the resonant frequency related to the mass/thickness of the film in contact with the surface and energy dissipation associated with the rheological properties of the adsorbed film, induced by adsorption/desorption processes or by structural changes produced within the thin film 68. The 4-sensor chambers of the Q-Sense E4 enable the performance of four simultaneous measurements under controlled experimental conditions (temperature, flow rate), increasing reproducibility and decreasing experiment duration 69. Also, different coatings (gold, silicon dioxide, titanium, hydroxyapatite) can be used, broadening the application field of the technique.
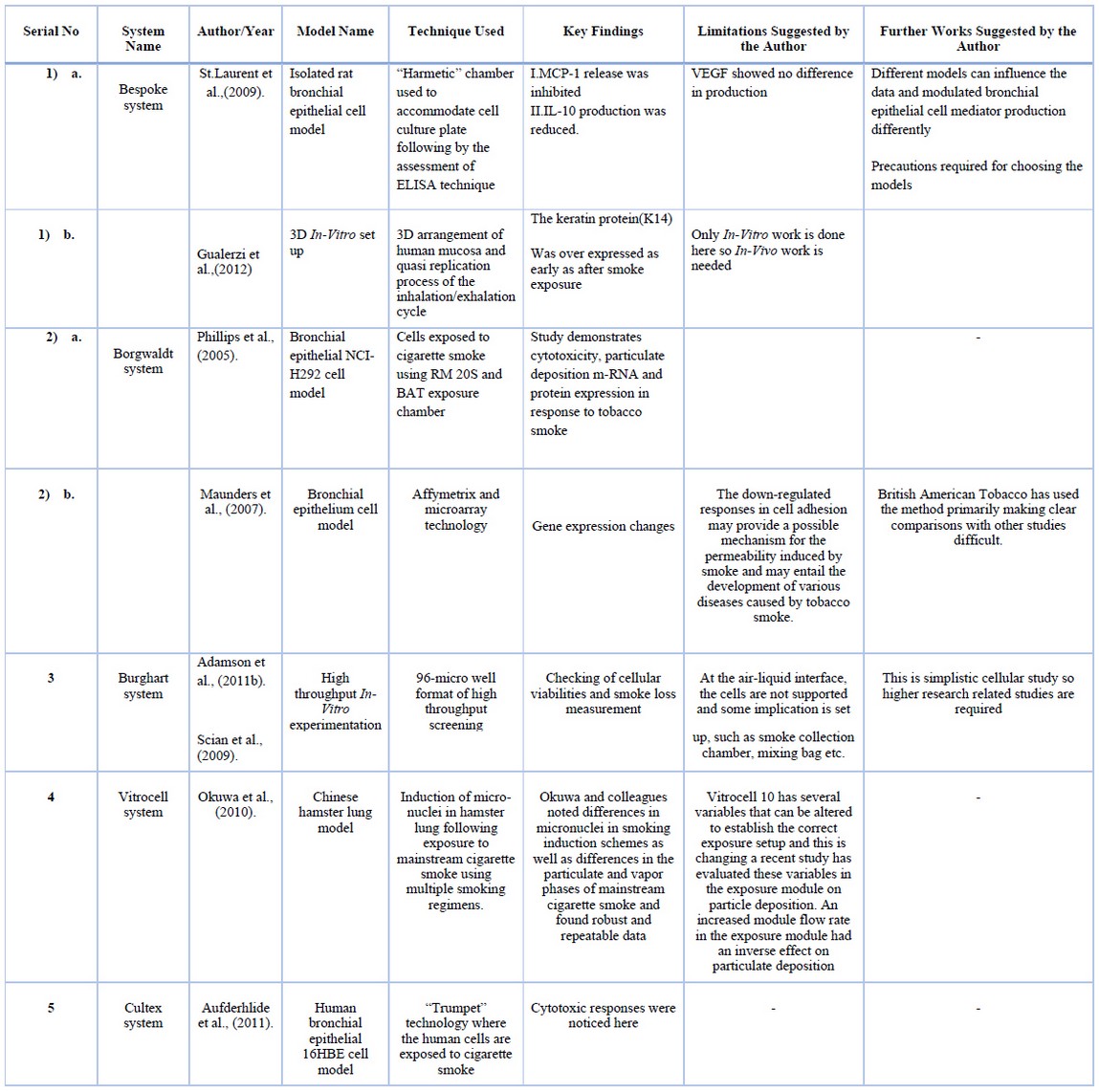
Table 8. Models with techniques, findings, limitations, and further suggestions.
The scientific literature covering whole smoke structures in vitro has provided a variety of related biological endpoints, illness, and toxicology. For instance, in vitro tobacco smoke has been shown to induce various cellular effects potentially associated with disease processes, including the up-regulation of a series of factors related to lung damage and inflammation 40. It has also been shown that tobacco smoke produces high levels of reactive oxygen species and oxidative stress that may cause cellular damage to lipids, proteins and DNA Moreover; it has been shown that cigarette smoking has several effects on gene expression in the human airways. Studies of bronchial epithelial cells obtained by bronchial brushing from smokers 'and non-smokers' airways have shown that cigarette smoke induces metabolizing and redox genes, tumor suppressor genes, and oncogenes alongside inflammatory process control 68.
DISCUSSION
Different smoke exposure systems have already been published in various investigative journals. With respiratory conditions such as asthma and COPD symptoms increasing worldwide, many new up-gradations associated with these subjection structures are launching in the commercial markets. Such severe respiratory diseases caused by inhalable pollutants, such as tobacco smoke, diesel exhaust, or other sources of pollution 70,71. Therefore, a substantial effect on national health systems is expected, indicating the long-term treatment, with routine tests accompanied by high costs 4,71. In this context, in vitro approaches can help develop more efficient protocols, such as evaluating new compounds' toxicity during the research and development process 56. This research will aim to better select substances suited to further development and a final reduction in the number of animal experiments 67. Hence, researchers have developed an in vitro exposure device that allows the examination of inhalable compounds for their pharmacological and toxicological effects. There are various options for using the different smoke exposure systems to produce a steady supply of cigarette smoke with slight variations over an extended period 71. A unique feature of the smoking systems is the conditioning and mixing chamber, which sets the primary concentration and provides a steady amount of "feed smoke" for the exposure components. Measuring and monitoring the flow rates to the exposure chambers is essential for controlling smoke levels. Any process or a mixture of measurements in the exposure portion is highly desirable for the continuous smoke concentration measurement 71. Carbon monoxide measurement can be an anchor measurement and can be reported as a record of exposure concentration over time. During treatment, measurement of different concentrations of smoke components should be carried out on a regular schedule 70,71. In an experiment involving the generation of smoke over prolonged periods, well-defined and documented exposure must be created. Smoke exposures are manpower-intensive and require continuous monitoring throughout the exposure cycle to maintain a steady supply of smoke 71.
Cleaning them regularly also is essential to prevent tar buildup. The smoking machine crashes and gets out of balance when pieces of tobacco and paper hit the sliding parts, and tar builds up 71. The efficiency will improve considerably as the user becomes more familiar with aligning and keeping the system clean. If carried out correctly, an experiment with a known exposure level will be conducted with good documentation of the smoke level, which is monitored very well from day 67. A great deal of advanced subjection structure for tobacco smoke is now used for a few days. Scientific knowledge has been published in various journals on these systems, and biological endpoints have been described. They are new to the research field and continue developing their presence 8,59.
CONCLUSION
Subjection structures for in vitro smoke occupy a role in fundamental and mechanical science. Various scientific literature showed the wealth of associated biological endpoints, disease, and toxicological data. Using such structures, multiple advances in vitro test methods are a promising sign and suggest that these devices can support and supplement several potential exposure scenarios. Depending on the equipment used, the ratio of smoke to air, the flow rate of mixing air added to the smoke diluter, and the fraction of smoke, in-vitro exposure systems can be viewed in many ways. Dosimetry is the most common and influential of all the methods for evaluating in-vitro tobacco smoke. The dosimetry method can bridge the gap and play an essential role in calculating and validating the actual cellular dosage. Different in-vitro models were developed based on exposure systems useful for evaluating tobacco smoke's biological activity. To understand tobacco smoke and disease and the mechanisms of disease and classify smoke toxicants responsible for severe medical conditions, in-vitro approaches will be necessary, ensuring the related exposure system is appropriately characterized.
Author Contributions: Moulima Das: Literature Review & Investigation, Software, Data Curator, Visualization, Graphs and Table preparation, Writing – Original Draft Preparation & corresponding author. Anupam Saha: Writing – Reviewing and Editing, Acquiring Permissions, Reformatting, Correction, Software and other essentials.
Permission: With reference to Medical Devices Rules, 2017 India, Rule no. 51 with regards to the conduct of academic study of Medical Devices this paper is made. Permission for copyright has been obtained for Fig No. 1 and Fig No. 2 (a) & (b) from concerned authority.
Funding: Authors declare that there is no such funding related to this article.
Acknowledgments: It is the wealth of experience and knowledge that is generated within the portals of NSHM Knowledge Campus, Group of Institutions-Kolkata, West Bengal, which enlightened my path to complete this manuscript. Authors would like to owe a special sense of gratitude and deep regards to the following people at Elsevier: David Throne and Jason Adamson. Without their support, we could never be able to complete this topic. The view and information of these authors have made substantial contributions to conceptions and designs have been involved in drafting the manuscript
Conflicts of Interest: Authors declare that there is no conflict of interest.
REFERENCES
1. C. Andreoli, D. Gigante, A. Nunziata : A review of in vitro methods to assess the biologcial activity of tobacco smoke with the aim of reducing the toxicology of smoke.Toxicology In Vitro, 17 (2003), pp. 587-594
2. M. Aufderheide, H. Gressmann: A modified Ames assay reveals the mutagenicity of native cigarette mainstream smoke and its gas vapour phase. Experimental and Toxicologic Pathology, 58 (2007), pp. 383-392
3. P.J. Barnes: New concepts in chronic obstructive pulmonary disease. Annual Review of Medicine, 54 (2003), pp. 113-129
4. J. Fowles, E. Dybing: Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tobacco Control, 12 (2003), pp. 424-430
5. M. Johnson, J. Schilz, M. Djordjevic, J. Rice, P. Sheilds: Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemioilogy, Biomarkers and Prevention, 12 (2009), pp. 3263-3304
6. H. Nara, Y. Fukano, T. Nishino, M. Aufderheide: Detection of the cytotoxicity of water-insoluble fraction of cigarette smoke by direct exposure to cultured cells at an air-liquid interface.Experimental and Toxicologic Pathology, 65 (2013), pp. 683-688
7. D. Ritter, J.W. Knebel, M. Aufderheide: Exposure of human lung cells to inhalable substances: a novel test strategy involving clean air exposure periods using whole diluted cigarette mainstream smoke. Inhalation Toxicology, 15 (2003), pp. 67-84
8. J. Adamson, D. Azzopardi, G. Errington, C. Dickens, J. McAughey, M. Gaça: Assessment of an in vitro whole cigarette smoke exposure system: the Borgwaldt RM20S 8-syringe smoking machine. Chemistry Central Journal, 5 (2011), p. 50
9. S. Bakand, C. Hayes: Troubleshooting methods for toxicity testing of airborne chemicals in vitro. Journal of Pharmacological and Toxicological Methods, 61 (2010), pp. 76-85
10. M.D. Johnson, J. Schilz, M.V. Djordjevic, J.R. Rice, P.G. Shields: Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol. Biomarkers Prev., 18 (2009), pp. 3263-3304
11. K. Okuwa, M. Tanaka, Y. Fukano, H. Nara, Y. Nishijima, T. Nishino: In vitro micronucleus assay for cigarette smoke using a whole smoke exposure system: a comparison of smoking regimens. Exp. Toxicol. Pathol., 62 (2010), pp. 433-440.
12. M.J. Scian, M.J. Oldham, D.B. Kane, J.S. Edmiston, W.J. McKinney: Characterization of a whole smoke in vitro exposure system (Burghart Mimic Smoker-01).Inhal. Toxicol., 21 (2009), pp. 234-243
13. Scian MJ, Oldham MJ, Kane DB, Edmiston JS, McKinney WJ: Characterization of a whole smoke in vitro exposure system (Burghart Mimic Smoker-01). Inhal Toxicol. 2009, 21: 234-243. 10.1080/08958370802482515.
14. Bernhard D, Huck CW, Jakschitz T, Pfister G, Henderson B, Bonn GK, Wick G: Development and evaluation of an in vitro model for the analysis of cigarette smoke effects on cultured cells and tissues. J Pharm Toxicol Meth. 2004, 50: 45-51. 10.1016/j.vascn.2004.01.003.
15. M. Aufderheide and U. Mohr, “CULTEX–a new system and technique for the cultivation and exposure of cells at the air/liquid interface,” Experimental and Toxicologic Pathology, vol. 51, no. 6, pp. 489–490, 1999.
16. Kaur N, Lacasse M, Roy JP, Cabral JL, Adamson J, Errington G, Waldron KC, Gaça MD, Morin A: Evaluation of precision and accuracy of the Borgwaldt RM20S smoking machine designed for in vitro exposure. Inhal Toxicol. 2010, 22: 1174-83. 10.3109/08958378.2010.533840.
17. Adamson J, Hughes S, Azzopardi D, McAughey J, Gaҫa M: Real-time assessment of cigarette smoke particle deposition in vitro. Chemistry Central Journal. 2012, 6: 98-10.1186/1752-153X-6-98.
18. Thorne D, Adamson A: A review of in vitro cigarette smoke exposure systems. Exp Toxicol Pathol. 2013, In Press.
19. Measurement Systems Analysis reference manual:Automotive Industry Action Group (AIAG). Daimler Chrysler Corporation, Ford Motor Company, General Motors Corporation: Supplier Quality Requirements Taskforce, Michigan, United States (2002)
20. S. Mülhopt, S. Diabate, T. Krebs, C. Weiss, H.R. Paur: Lung toxicity determination by in vitro exposure at the air liquid interface with an integrated online dose measurement. J. Phys.: Conf. Ser., 170 (2009), p. 012008
21. M. Thompson, S.L.R. Ellison, A. Fajgelj, P. Willetts, R. Wood: Harmonised guidelines for the use of recovery information in analytical measurement (technical report). Pure Appl. Chem., 71 (1999), pp. 337-348
22. D.J. Wheeler: EMP III. Evaluating the Measurement Process & Using Imperfect Data. SPC Press Statistical Process Controls, Inc., Knoxville, Tennesse (2006), ISBN 978-0-945320-67-8
23. S.L.R. Ellison, V.J. Barwick, T.J. Duguid Farrant: Practical Statistics for the Analytical Scientist: A Bench Guide.(second ed.), RSC Publishing (2009), ISBN: 978-1-84755-955-5
24. U. Deschl, J. Vogel, M. Aufderheide: Development of an in vitro exposure model for investigating the biological effects of therapeutic aerosols on human cells from the respiratory tract. Exp. Toxicol. Pathol., 63 (2011), pp. 593-598
25. Clunes L, Bridges B, Alexis N, Tarran R: In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol. 2008, 32: 201-207. 10.1093/jat/32.3.201.
26. Zhang W, Case S, Bowler RP, Martin RJ, Jiang D, Chu W: Cigarette smoke modulates PGE(2) and host defence against Moraxella catarrhalis infection in human airway epithelial cells. Respirology. 2011, 16: 508-516. 10.1111/j.1440-1843.2010.01920.x.
27. Gualerzi A, Sciarabba M, Tartaglia G, Sforza C, Donetti E: Acute effects of cigarette smoke on three-dimensional cultures of normal human oral mucosa. Inhal Toxicol. 2012, 24: 382-389. 10.3109/08958378.2012.679367.
28. De Serres F, Shelby M: Recommendations on data production and analysis using the Salmonella/microsome mutagenicity assay. Mutat Res. 1979, 64: 159-165. 10.1016/0165-1161(79)90101-8.
29. Newland N, Richter A: Agents associated with lung inflammation induce similar responses in NCI-H292 lung epithelial cells. Toxicol In Vitro. 2008, 22: 1782-1788 10.1016/j.tiv.2008.07.009.
30. Phillips J, Kluss B, Richter A, Massey E: Exposure of bronchial epithelial cells to whole cigarette smoke: assessment of cellular responses. Altern Lab Anim. 2005, 33: 239-248.
31. M. Aufderheide and U. Mohr, “A modified CULTEX system for the direct exposure of bacteria to inhalable substances,” Experimental and Toxicologic Pathology, vol. 55, no. 6, pp. 451–454, 2004.
32. K. de Bruijne, S. Ebersviller, K. G. Sexton et al., “Design and testing of Electrostatic Aerosol In Vitro Exposure System (EAVES): an alternative exposure system for particles,” Inhalation Toxicology, vol. 21, no. 2, pp. 91–101, 2009.
33. C. I. Grainger, L. L. Greenwell, D. J. Lockley, G. P. Martin, and B. Forbes, “Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier,” Pharmaceutical Research, vol. 23, no. 7, pp. 1482–1490, 2006.
34. C. Schmalz, H. G. Wunderlich, R. Heinze, F. H. Frimmel, C. Zwiener et al., “Application of an optimized system for the well-defined exposure of human lung cells to trichloramine and indoor pool air,” Journal of Water and Health, vol. 9, no. 3, pp. 586–596, 2011.
35. D. Wijte, M. J. Alblas, D. Noort, J. P. Langenberg, and H. P. van Helden, “Toxic effects following phosgene exposure of human epithelial lung cells in vitro using a CULTEX system,” Toxicology in Vitro, vol. 25, no. 8, pp. 2080–2087, 2011.
36. S. Mülhopt, H. R. Paur, S. Diabate, and H. F. Krug, “In-vitro testing of inhalable fly ash at the air liquid interface,” in Environmental Monitoring, Y. J. Kim, Ed., pp. 402–414, Springer, Dordrecht, The Netherlands, 2008.
37. Maron D, Ames B: Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983, 113: 173-215. 10.1016/0165-1161(83)90010-9.
38. De Serres F, Shelby M: Recommendations on data production and analysis using the Salmonella/microsome mutagenicity assay. Mutat Res. 1979, 64: 159-165. 10.1016/0165-1161(79)90101-8.
39. Stratton K, Shetty P, Wallace R, Bondurant S: Clearing the smoke: the science base for tobacco harm reduction-executive summary. Tob Control. 2001, 10: 189-195. 10.1136/tc.10.2.189.
40. T. Adam, J. McAughey, C. McGrath, C. Mocker, R. Zimmermann: Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Analytical and Bioanalytical Chemistry, 394 (2009), pp. 1193-1203
41. J. Adamson, D. Thorne, A. Dalrymple, D. Dillon, C. Meredith: Cigarette smoke deposition in a Vitrocell® exposure module: real-time quantification in vitro using quartz crystal microbalances. Chemistry Central Journal, 7 (2013), p. 50
42. R.R. Baker: The generation of formaldehyde in cigarettes – overview and recent experiments. Food and Chemical Toxicology, 44 (2006), pp. 1799-1822
43. M. Borgerding, H. Klus: Analysis of complex mixtures – cigarette smoke. Experimental and Toxicologic Pathology, 57 (2005), pp. 43-73
44. K. Bruijne, S. de Ebersviller, K.G. Sexton, S. Lake, D. Leith, R. Goodman, et al. Design and testing of electrostatic aerosol in vitro exposure system (EAVES): an alternative exposure system for particles. Inhalation Toxicology, 21 (2) (2009), pp. 91-101
45. M.S. Cooke, M.D. Evans, M. Dizdaroglu, J. Lunec: Oxidative DNA damage: mechanisms, mutation, and disease. Federation of American Societies for Experimental Biology Journal, 17 (2003), pp. 1195-1214
46. Y. Fukano, M. Ogura, K. Eguchi, M. Shibagaki, M. Suzuki: Modified procedure of a direct in vitro exposure system for mammalian cells to whole cigarette smoke. Experimental and Toxicologic Pathology, 55 (2004), pp. 317-323
47. N.R. Hackett, R. Shaykhiev, M.S. Walters, R. Wang, R.K. Zwick, B. Ferris, et al. The human airway epithelial basal cell transcriptome. PLoS ONE, 6 (2011), p. e18378
48. N.E. Klepeis, W.R. Ott, P. Switzer: Real-time measurement of outdoor tobacco smoke particles. Journal of Air Waste Management Association, 57 (2007), pp. 522-534
49. J.C.-J. Lin, R. Jean-Phillippe, J. Verreault, S. Talbot, F. Cote, R. Couture, et al. An ex vivo approach to the differential parenchymal responses induced by cigarette whole smoke and its vapor phase. Toxicology, 293 (2012), pp. 125-131
50. E. Uttenthaler, C. Koesslinger, S. Drost: Characterization of immobilization methods for African swine fever virus protein and antibodies with a piezoelectric immunosensor. Biosensors and Bioelectronics, 13 (1998), pp. 1279-1286
51. M. Borgerding, H. Klus: Analysis of complex mixtures – cigarette smoke. Experimental and Toxicologic Pathology, 57 (2005), pp. 43-73
52. R.N. Grass, L.K. Limbach, E.K. Athanassiou, W.J. Stark: Exposure of aerosols and nanoparticle dispersions to in vitro cell cultures: a review on the dose relevance of size, mass, surface and concentration. Journal of Aerosol Science, 41 (2010), pp. 1123-1142
53. L.E. Haswell, K. Hewitt, D. Thorne, A. Richter, M. Gaça: Cigarette smoke total particulate matter increases mucous secreting cell numbers in vitro: a potential model of goblet cell hyperplasia. Toxicology In Vitro, 24 (2010), pp. 981-987
54. S. Heeg, M. Doebele, W.A. Von, O.G. Opitz: In vitro transformation models: modeling human cancer. Cell Cycle, 5 (2006), pp. 630-634
55. W. Hofmann, R. Winkler-Heil, J. McAughey: Regional lung deposition of aged and diluted sidestream tobacco smoke. Journal of Physics (2009)
56. IARC, International Agency for Research on Cancer. Monographs on the evaluation of the carcinogenic risk of chemicals to humans, allyl compounds, aldehydes, epoxides and peroxides. World Health Organization, Lyon, France (1985)
57. IARC, International Agency for Research on Cancer. Chromium and chromium compounds
58. Some metals and metallic compounds. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, World Health Organization, Lyon, France (1990)
59. IARC, International Agency for Research on Cancer. Beryllium, cadmium, mercury and exposures in the glass manufacturing industry. IARC monographs on the evaluation of carcinogenic risks to humans. World Health Organization, Lyon, France (1993)
60. C. Liu, K. McAdam, A. Perfetti: Some recent topics in cigarette smoke science. Mini-Reviews in Organic Chemistry, 8 (2011), pp. 349-359
61. Rushton, E.K. , Jiang, J. , Leonard, S.S. , Eberly, S. , Castranova, V. , Biswas, P. , Elder, A. , Han, X. , Gelein, R. , Finkelstein, J. , & Oberdörster, G. (2010). Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. Journal of Toxicology & Environmental Health A 73, 445–461.
62. Misko TP, Schilling RJ. Salvemini D, et al. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 1993;214:11–16.
63. C. Persoz, S. Achard, C. Leleu, I. Momas, N. Seta: An in vitro model to evaluate the inflammatory response after gaseous formaldehyde exposure of lung epithelial cells. Toxicology Letters, 195 (2010), pp. 99-105
64. C. O'Sullivan, G. Guilbault: Commercial quartz crystal microbalances: theory and applications. Biosensors and Bioelectronics, 14 (1999), pp. 663-670
65. Chambers DC, TunnicliVe WS, Ayres JG. Acute inhalation of cigarette smoke increases lower respiratory tract nitric oxide concentrations. Thorax 1998;53:677–9.
66. M. Savi, M. Kalberer, D. Lang, M. Ryser, M. Fierz, A. Gaschen, et al. A novel exposure system for the efficient and controlled deposition of aerosol particles onto cell cultures. Environmental Science and Technology, 42 (2008), pp. 5667-5674
67. Miles A, Bohle DS, Glassbrenner PA, et al. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem 1996;271:40–7.
68. J. Adamson, D. Thorne, J. McAughey, D. Dillon, C. Meredith: Quantification of cigarette smoke particle deposition in vitro using a triplicate quartz crystal microbalance exposure chamber. BioMed Research International (2013)
69. Rahman, W. MacNee: Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. American Journal of Physiology – Lung Cellular and Molecular Physiology, 277 (1999), pp. L1067-L1088
70. Rutgers SR, van der Mark ThW, Coers W, et al. Markers of nitric oxide metabolism in sputum and exhaled air are not increased in chronic obstructive pulmonary disease.Thorax 1999;54:576–80.
71. Gualerzi, M. Sciarabba, G. Tartaglia, C. Sforza, E. Donetti: Acute effects of cigarette smoke on three-dimensional cultures of normal human oral mucosa. Inhalation Toxicology, 24 (2012), pp. 382-389.
Received: 10 June 2021 / Accepted: 24 January 2022 / Published: date. 15 May 2022
Citation: Das, Moulima.; Saha, Anupam., Probe on Various Experimental Cigarette Smoke Subjection Structure. Revis Bionatura 2022;7(2). 7. http://dx.doi.org/10.21931/RB/2022.07.02.7
