2023.08.02.74
Files > Volume 8 > Vol 8 No 2 2023
Study
of specific activities of phenolic compounds produced from Fungi Pleurotus
Ostreatus and Agaricus Bisporus

Mallak
M. Ammar1,*
1 Collage Basic Education, Al-Mustansiriah University,
Iraq
*
Correspondence: [email protected]
Available
from: http://dx.doi.org/10.21931/RB/2023.08.02.74
ABSTRACT
Specific
activities of phenolic compounds produced from the fruiting bodies of the fungi
PO and AB (the locally produced strain in Iraq) obtained from the Department of
Plant Protection - Department of Organic Agriculture - Baghdad - Iraq were studied.
Then, the phenolic compounds were determined after cleaning, drying and
grinding the fruiting bodies. The alcoholic extract of the two fungi was
prepared by adding 0.2 g of fruiting bodies powder per (1) ml of 98% ethyl
alcohol, where the mixture was filtered, concentrated and kept in the
refrigerator until use. The aqueous extract was prepared by adding 25 g of
fruiting bodies powder per 500 ml of boiled distilled water. Similarly, the
mixture was mixed, filtered and stored in the refrigerator until use. The study
findings confirmed the existence of significant differences at the probability
level (P ˂ 0.05) for the alcoholic and aqueous extracts and for the fungi in
the content of phenolic compounds.
Conversely,
the reducing power was increased by increasing the concentrations prepared for
the extracts prepared from the two fungi under study. The free radical
scavenging method DPPH was used to estimate the antioxidant activity of
alcoholic and aqueous extracts and of the fungi PO and AB. Thus, the results
showed the superiority of ethyl acetate extract compared to the other used
solvents.
Keywords:
Edible
fungi, phenolic compounds, reducing power, free radical scavenging.
INTRODUCTION
Food additives have
always dominated the debate of researchers interested in human health due to
their close relationship with most diseases, especially cancer, allergies and
other diseases 9. Antioxidants are considered one of the essential
food additives used to preserve food products and extend their storage life 18.
Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated
hydroxytoluene (BHT) were used to prevent the oxidation of fats in food.
However, their use was limited because they produced carcinogenic substances
during decomposition, confirmed by the latest studies 1. The need
has increased to replace industrial antioxidants with others from natural
sources such as plants, microorganisms and fungi with fruiting bodies. These
sources are safe for health and help the body reduce oxidative damage,
especially phenolic compounds 19. The phenolic compounds taken from
natural sources are among the most important compounds that can search for free
radicals that cause oxidation 2. Pleurotus ostreatus (PO) and
Agaricus bisporus (AB) are good sources of vitamins, minerals, proteins,
carbohydrates and unsaturated fatty acids such as oleic acid, linoleic acid,
fiber, fats and sodium 5. Therefore, it is considered a healthy food
for people with high cholesterol and high blood pressure 6.
Researchers Cheung M. and Cheung K confirmed that edible fungi, including PO
and AB, contain biologically active molecules capable of collecting free
radicals from lipid oxidation, which causes most health problems. The most
important of these molecules are polysaccharides, phenols, and vitamins such as
vitamins A and C, and beta-carotene found in the above fungi are among the most
important biologically active compounds used as antioxidants 10.
Consequently, there was an urgent need to study the importance of some of the
phenolic compounds that can be produced from the fungi PO and Ab, which are
produced locally from our dear country, Iraq.
MATERIALS AND
METHODS
Fruiting bodies of
the two fungi were obtained from the Department of Plant Protection -
Department of Organic Agriculture - Baghdad, Iraq. The fruiting bodies were
thoroughly cleaned with distilled water, then wiped with a clean cloth and
placed in a thermal oven at 40-45 °C for one day. The samples were ground with
an Egyptian electric grinder from Tornado Company. The powder was then passed
through a sieve whose holes are about 0.5 mm in diameter. The resulting powder
was packed into polyethylene bags, and the bags were completely emptied of air
and stored until use 20.
Preparation of
alcoholic and aqueous extract of the fungi under study
The alcoholic
extract of the two fungi PO and AB was prepared separately according to the
method of 16, as (0.2) g of the prepared powder was added to every 1
ml of ethyl alcohol 98% and mixed by a magnetic stirrer and left for 24 hours
at 25°C. The extract was then filtered using a filter paper (0.1NO), and the filtrate
was concentrated at a temperature of 40°C using a rotary vacuum evaporator.
Then the resulting substance was then kept in opaque and airtight bottles and
in the refrigerator until use. As for the aqueous extract was prepared
according to the method of 3, as 25 g was added to every 500 ml of
boiled distilled water. The mixture was stirred for 30 minutes with a magnetic
stirrer and filtered with a Buchner funnel. Furthermore, the excess water was
disposed of by a rotary evaporator at a temperature of 50 °C, and the filtrate
was left at 25 °C to dry and kept in the refrigerator until use.
Determination of
total phenolic compounds
According to the
method of 8, in preparing the standard curve for gallic acid, a
stock solution was prepared by adding 0.125 g of it to 100 ml of methanol at a
concentration of 50%. Different volumes of the prepared solution were taken in
a set of test tubes, and 50% methanol was added to it so that the volume was
0.5 ml, as shown in Table 1. Then, Folin and Na2CO3 75%
reagent were added with a volume of 2.5 and 2 ml, respectively, so that the
total volume of the final solution was 5 ml. The absorbance was recorded by a
spectrophotometer at a wavelength of 760 nm.
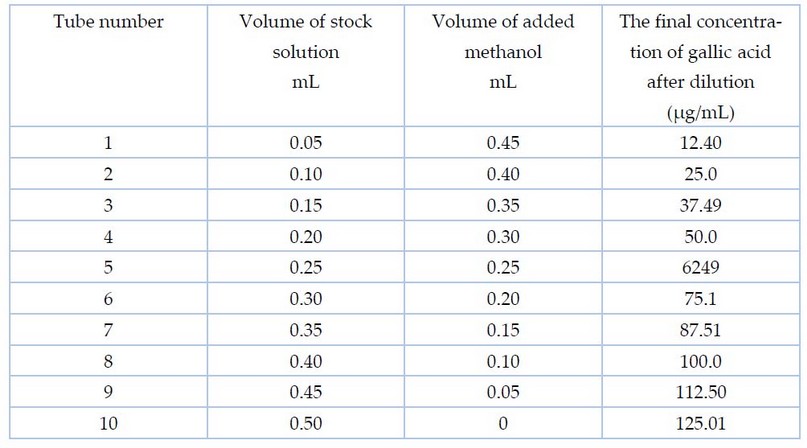
Table
1. The
concentrations and volumes used to prepare gallic acid

Figure
1.
Graph of the standard curve of gallic acid
Preparation
of alcoholic and aqueous extracts of fungi PO and AB for the determination of
phenolic compounds
2.5
ml of Folin reagent was added to 0.5 ml of fungal filtrate for each fungus
under study separately and left for 10 minutes at a temperature of 25 °C, then
Na2CO3 75% was added. The mixture was placed at 40 °C for
half an hour, and the absorbance was measured at a wavelength of 760 nm.
Measuring the reducing power of alcoholic and
aqueous extracts and of the fungi under study by 11 mixed (1) ml of
each section with 2.5 solutions of 1% potassium ferricyanide and adding 2.5 ml
of 0.2 M phosphate buffer solution at PH (6.6). Then, the mixture was kept at a
temperature of 50 °C for a third hour; later, trichloro acetic acid chloride
(TAC) was added at a concentration of 10% and placed in a centrifuge (1900 x g)
for 10 minutes. (2.5) ml of the filtrate were taken with (2.5) ml of distilled
water and (0.5) ml of ferric chloride (0.1%). A control sample was prepared by
preparing all the materials above to measure the reducing force, except they
were free of the extracts under study. Moreover, the absorbance was measured at
a wavelength of 700 nm after leaving it for half an hour. According to the
following law:

Where:
F = reducing power, X = absorbance reading of the control sample, Y = absorbance
reading of the model.
DPPH radical-scavenging
A
0.1 mmol of 1,1-Diphenyl-2-picryl hydrazyl (DPPH) solution was mixed after
dissolving it in ethanol at a concentration of 95% with (1) ml of the extract.
The mixture was mixed away from light at a temperature of 25°C for a full hour
until the purple color disappeared and a violet or light pink color appeared,
which was determined by measuring the decrease in absorbance at a wavelength of
517 nm. The following equation was applied:
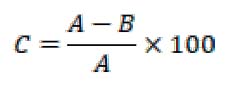
Where:
C = free radical scavenging DPPH, A = absorbance for the control treatment, B =
absorbance of the mixture.
The
efficiency of ethyl alcohol extract in scavenging free radicals was compared
with other solvents, such as hexane, chloroform, n-butanol, and ethyl acetate.
RESULTS
Determination
of total phenolic compounds
The
results shown in Table 2 and Figure 2 showed that there were significant
differences at the probability level (p˂0.05) for the alcoholic and aqueous
extracts of the two fungi PO and AB in the content of phenolic compounds, as it
reached for the alcoholic extract of the two fungi (5.79 and 6.9) mgGAE/g
respectively. As for the aqueous extract of the two fungi, the number of
phenolic compounds was 6.12 and 7.01 mgGAE/g, respectively. These results are
consistent with 4 when they concluded that the total phenols of the
aqueous extract of PO and AB were 6.27 and 7.60 mgGAE/g.

Table
2. The
total amount of phenols for the fungi under study and the alcoholic and aqueous
extracts

Figure 2. The
total amount of phenols for the fungi under study and the alcoholic and aqueous
extracts
Determination of the reducing
power
Figure 3 shows the reducing power
of alcoholic and aqueous extracts of fungi PO and AB prepared at concentrations
of 100, 80, 60, 40, 20, and 10 mg/ml for each sample. It is noted that the
reducing power increased with increasing concentration. The concentration (100
mg/ml) showed the highest reducing power in all alcoholic and aqueous extracts,
as the PO alcoholic extract had the highest reducing power of 185.9% at a
concentration of 100 mg/ml. It was observed that the capacity of reducing the
power of the aqueous extract of fungus PO is 162.03%. In comparison, the
alcoholic and aqueous extract of the fungus AB recorded a reducing power of
149.3 and 132.02% for the alcoholic and aqueous extracts, respectively. As for
the reducing power of BHA, it was recorded at 190.45, and the results of the
statistical analysis recorded this, as it showed the superiority of alcoholic
extracts over aqueous extracts with the same concentration.
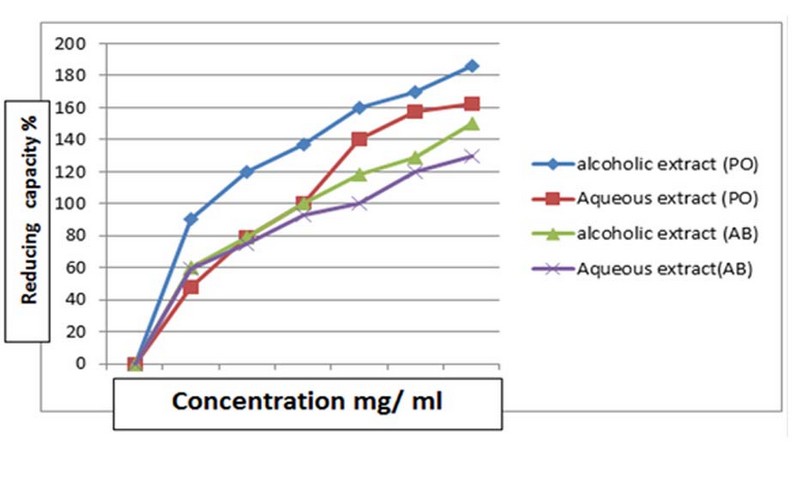
Figure 3. The
reducing capacity of alcoholic and aqueous extracts of PO AB in comparison with
BHT
Determination of the antioxidant
activity by DPPH free radical scavenging method
Figure 3 shows the superiority of
ethyl acetate extract in free radical scavenging compared to other solvents of
the two fungi, as the scavenging % of PO and AB was about 65.5% and 61.01%)
respectively. However, it amounted to 31.5, 25.8 and 20.1% for butanol,
chloroform and hexane extracts of the fungus PO, respectively. As for AB
extract, the percentage of free radical scavenging was about 28.9%, 22.01 %,
and 17.9%, respectively.

Table
3. Statistical
analysis for determination of the antioxidant activity by DPPH free radical
scavenging method for PO fungi extract
The statistical analysis result
showed significant differences at the level (0.05) between ethyl extracts and
among other extracts, as shown in Table 4.
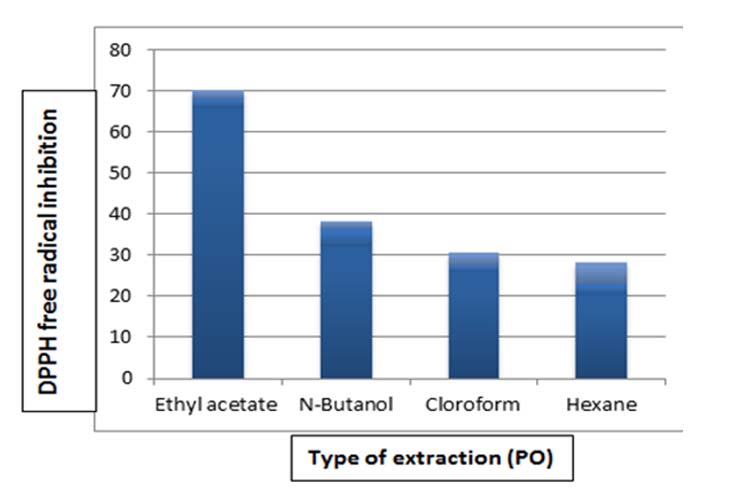
Figure 4.
Determination of the antioxidant activity by DPPH free radical inhibition
method for PO extract
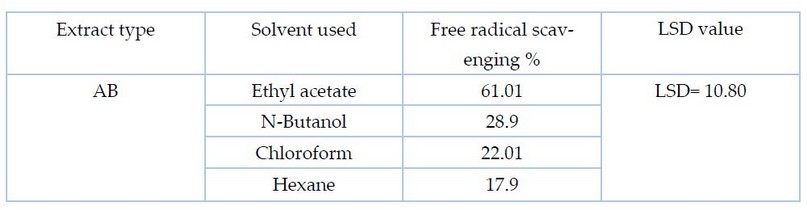
Table
4.
Statistical analysis to determine the antioxidant activity by the method of
inhibiting free radical DPPH for the AB extract
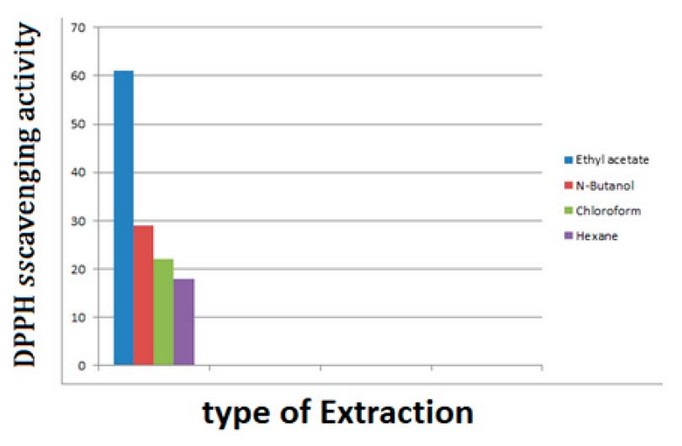
Figure 5.
Statistical analysis to determine the antioxidant activity by DPPH free radical
inhibition method for AB extract
The analysis of these results explained that
these extracts contain antioxidant compounds that contain hydroxyl groups that
give hydrogen atoms capable of interacting with free radicals, turning these
radicals into more stable substances, thus ending the reaction chain of the
free radical.
The difference in the number of phenolic
compounds between the aqueous and alcoholic extracts may be due to the polarity
of all the solvents used in the extraction and the chemical nature of the
separated compounds. These results are consistent with what was reached by 4.
However, 14 indicated that most phenolic compounds are dissolved in
water as sugar-linked glycosides formed in the cell walls and contain active
hydroxyl groups. The concentrations of phenolic compounds in the filtrate were
calculated and compared to the standard curve for Gallic acid 7. The
superiority of alcoholic extracts over aqueous extracts with the same
concentration. This may be due to the efficiency of the solvents used to
extract the active compounds 17. (Yang et al., 2020) explained that
the reason is the possibility of combinations called reducing compounds that
can interact with free radicals to convert them to more stable products and
then end the free radical reaction chain. Figure 4 shows that the DPPH-free revolutionary
scavenging method determines the antioxidant activity of alcoholic and aqueous
extracts. It is one of the critical analyses to reveal the ability of the
extract to prevent oxidation in the promotion stage by neutralizing or
preventing free radical formation 12. Finally, the results were
confirmed by 15 that the group that donates the electron radical
increases the retention of free radicals and that the independent group of the
electron reduces the effectiveness of holding these radicals.
CONCLUSIONS
The fruiting bodies of the two fungi PO and
AB, produced locally in Iraq, contain phenolic compounds that industrial
phenolic compounds can replace.
We are increasing the reducing power capacity
by increasing the concentration of the extracts prepared in the current
research.
Ethyl acetate extract had the most
significant effect compared to the solvents used in the research.
RECOMMENDATIONS
The production of natural antioxidants from
edible fungi is guaranteed and does not contain side effects instead of
synthetic antioxidants.
Acknowledgments
I want to thank Al-Mustansiriya University
and the College of Basic Education for their assistance in conducting the
current research.
REFERENCES
1.
Abdel-Hameed,
E. Total phenolic contents and free radical scavenging activity of certain
Egyptian Ficus species leaf samples.Food Chem. 2009 ;(114).
1271-1277.
2.
Aby,
K.; Hvattum, E. and Skrede, G. Analysis of flavonides and other phenolic
compounds using high-performance liquid chromatography with coulometer array
detect in relationship to antioxidant activity. J. Agric. Food Chem. 2004;
52: 4595-4603.
3.
Al-Musawi
, O and Al-Halfi,S. Extraction of
phenolic compounds from some vegetables and estimation of their antioxidant
activities. Basra Journal of Agricultural Sciences . 2011;( 24
) .Issue (1) .
4.
AlispahiC,
A., Sapcanin, A., Salihovic, M., Ramic, E., Dedic, A., Pazalja, M. Phenolic
content and antioxidant activity of mushroom extracts from Bosnian market. Bulletin
of the Chemists and Technologists of Bosnia and Herzegovina. 2015; (944)
: (5 -8)
5.
Ammar,M.
Mallak. utilization of Agaricus bisporus to inhibit the growth of some
microorganism species. Plant Archives. 2019; 19, Supplement
2, 2019 pp. 627-630.
6.
Ammar,M.
Mallak. Evaluation in Inhibiting Growth of Some Lentinula edode Bacteria
Causing Urinary Tract Infection. Indian Journal of Ecology (48 )Special
Issue. 2021; (13): 264-267.
7.
Arora,
S. & Chandra, P . Assay of antioxidant potential of two Aspergillus
isolates by different methods under various physio-chemical conditions. Brazilian
Journal of Microbiology. 2010; 41(3). (765-777).
8.
Ayoola,
G.; Ipav, S.;Sofidiya,M.O.;AdepojuBeello, A.;Coker, A.
Odugbemi,T.O.Phytochmical Screening and Free Radical Scavenging Activities of
the Fruits and Leaves of Allanblackia floribuna Oliv (Guttiferae) . International Journal of Health Research, 2008;1(2):87-93
9.
Bancil,
S.; Sandall, M.; Rossi, M.; Chassaing,
B.; Lindsay, J.; Whelan, K. Food
additive emulsifiers and their impact on gut Microbiom; Permeability and
inflammation. Mechanistic Insights in Inflammatory Bowel Disease Get access to
Arrow. Journal of Crohn's and Colitis. 2010; 15 (6):1068–1079.
10.
Barros
L, M, Queirós B, and Baptista, P. Total
phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible
mushrooms and their antioxidant activities. Fd Chem. 2007; (103).
413 ــ419.
11.
Benzie,
F. and Strain, J . The ferric-reducing ability of plasma (FRAP) is a measure of
antioxidant power in the FRAP assay.Anal Biochem. 1996; 239
(70-76).
12.
Bind,
A ; Singh, K ; Prakash, V ; and
Kumar, M. Evaluation of
antioxidant through solid state fermentation from pomegranate peels using
Aspergillus niger and its antibacterial properties. International Journal of
Pharmacy and Biological Science. 2014; 4 (1): 104 – 112.
13.
Cheung
, M and Cheung ,K . Mushroom extracts with antioxidant activity against lipid
peroxidation. Fd Chem. 2005; 89 (3). 403 Jــ
409.
14.
Devasagayam,
A and Sanis,B. Immune System and
Antioxidants, Especially Those Derived From Indian Medicinal Plants, Indian
Journal. Exper. Biol. 2002; (40) 639 - 655.
15.
Farhoosh , R.; Gholam
, A.; Mohammad , G. & Khodaparast, H. Antioxidant activity of
various extracts of old tea leaves and black tea wastes. Food Chemistry.
2006; (100) : (231-236) .
16.
Guilin,
I; Oktay, M; Kireşci, O and Kufrevioglu.
Screening of antioxidant and antimicrobial activities of anise (Pimpinella
anisum L.) seed extracts. Food Chem. 2004; (83): 371ـ 382.
17.
Huang,
D , Chen, H. and Lin,Y. Antioxidant and
antiproliferative activities of sweet potato (Ipomoea batata L.) Lam (Tainong
57) constituents. Bot. Bull. Acad. Sin. 2019; (45) :179 -186.
18.
Singh,
R.; Sharma, S., and Singh, P.
Antioxidants: Their Health Benefits and
Plant Sources. Journal of Natural Products and Medicine. 2017; 4
(11): 23–26.
19.
Takaidza
, S; Mtunzi, F; & Pillay, M. Analysis of the phytochemical contents and
antioxidant activities of crude extracts from Tulbaghia species. Journal
of Traditional Chinese Medicine. 2018; 38
(2): 272ــ
279.
20.
Yim,
H.; Chye, F.; Tan, C.; Ng Y, C. and HoC, W. Antioxidant activities and total
phenolic content of aqueous Pleurotus ostreatus (cultivated oyster mushroom)
extract. Malaysian Journal of Nutrition. 2010.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published:
June 15, 2023
Citation: Ammar,
M.M. Study of Specific Activities of Phenolic Compounds Produced from Fungi Pleurotus
Ostreatus and Agaricus Bisporus. Revis Bionatura 2023;8 (2) 74. http://dx.doi.org/10.21931/RB/2023.08.02.74