2023.08.02.15
Files > Volume 8 > Vol 8 No 2 2023
New dimeric complexes with semicarbazone mannich-based ligand; formation, structural investigation and biological activity
Department of Chemistry, College of Education for Pure Science (Ibn Al-Haitham), University of Baghdad, Adhamiyah, Baghdad, Iraq
*Corresponding author; Emails: [email protected];
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.15
ABSTRACT
The formation of a new semicarbazone Schiff-base ligand (E)-2-(2-(phenyl(2-phenylhydrazinyl)- methyl)cyclohexylidene)hydrazine-1-carboxamide (HL) and its complexes are reported. The new ligand was prepared from the condensation of the Mannich-base 2-(phenyl(2-phenylhydrazinyl)methyl)cyclohexan-1-one (M) with the semicarbazide. A series of metal complexes were prepared by the reaction of the ligand with the metal chlorides of Cr(III), Mn(II), Co(II), Ni(II), Cu(II)), Zn(II) and Cd(II). The structure of the ligand and its complexes were elucidated through analytical and spectroscopic techniques. The analyses indicated the ligand behaves as a monobasic tridentate species and the isolation of dimeric complexes with the general formula; [Cr(L)(Cl)2(H2O)]2, [M(L)Cl]2 (where M= Mn(II), Co(II), Ni(II), Cu(II)), Zn(II) and Cd(II)). These studies revealed a distorted octahedral geometry for Cr(III), a distorted square planar for Cu(II) and a tetrahedral arrangement for Mn(II), Co(II), Ni(II), Zn(II) and Cd(II). The biological activity of the prepared compounds against Escherichia coli, Pseudomonas auroginosa, Staphylococcus aureus and Bacillus subtilis compared with Cefotaxime (as a standard antibiotic) was also explored. Some of the examined compounds indicated a similar antibacterial activity to Cefotaxime. Furthermore, antifungal activity against Candida albicans and Rhizopus sporium was investigated, which showed that the ligand and its complexes exhibited good antifungal activity.
Keywords: Schiff-base ligand; Structural characterization; Dimeric semicarbazone complexes; Biological activity.
INTRODUCTION
Mannich bases are one of the necessary reagents that play an essential role in developing synthetic organic chemistry. Mannich approach is a one-pot multicomponent reaction. This type of reaction represents a feasible procedure for highly reactive intermediates that can be quickly transformed into various new compounds 1. These include their role as precursors for synthesizing a range of natural product compounds and forming heterocyclic phenanthridine derivatives 2,3. Further, a variety of exciting ligands and, subsequently, their metal complexes have been derived from Mannich compounds 4. Mannich base ligands and their complexes are of great interest as they have a range of applications, including their uses in medicine 5 and as potential pharmaceutical agents 6. Further, they exhibit applications in polymer and surfactant chemistry, as detergent additives, and as antioxidants 7. Upon using ligands derived from Mannich bases as complexation agents, the biological activities of these complexes are enhanced 8. Schiff bases are important species that are used as complexation agents for metal ions. This is because they can form stable compounds with almost all metal ions. These compounds have applications in medicine, as a mimic of bioactive molecules, in catalysis, in analytical and environmental chemistry and as a scavenger for removing heavy metal ions 9. Their bioavailability makes these materials excellent antiviral, antiinflammatory, antiapoptotic and antibacterial agents 10,11. Semicarbazones are a Schiff base compound bearing N, O-chelating site for complexation 12. They can bind transition and non-transition ions and have a range of exciting applications, including their pharmaceutical and biological activities 12,13. Semicarbazone Schiff base compounds have a range of applications, including their ability to bind with DNA and act as antibacterial, antifungal, anticancer, antiinflammatory, antioxidant, antiviral, analgesic, and anticonvulsant agents 14-16. In the present work, we report the synthesis, structural characterization and biological behavior (antibacterial and antifungal influence) of a new semicarbazone ligand and its dimeric complexes. The ligand is designed to incorporate phenylhydrazine within the framework of the Mannich base that is used as a precursor for the preparation of the ligand. The aim of using phenylhydrazine in the formation of the ligand is (i) to explore the impact of this segment on the structural and coordination mode upon the reaction of the ligand with metal ions and (ii) to observe the influence of the phenylhydrazine on the biological activity of the prepared compounds against microbiological species.
MATERIALS AND METHODS
All reagents used in this work were commercially available and used as received. The mass spectrum for the ligand was measured using the electrospray technique (positive mode) on the Agilent mass spectrometer Sciex ESI mass analysis. The 1H-NMR for the ligand was recorded in DMSO-d6 using a Brucker 400 MHZ with a tetramethylsilane (TMS) as an internal reference. A Shimadzu Fourier Transform Infrared Spectrometer (FTIR-600) was used to record the spectra of compounds using KBr and CsI discs from 4000–250 cm-1. Electronic spectra were measured from 200–1100 nm for 10-3 M solutions in DMSO at room temperature with a Shimadzu 160 spectrophotometer. Melting points were determined using an electrothermal Stuart apparatus, model SMP40. Elements analysis (CHN) for ligand and their metal complexes were performed on an Eager 300 for EA1112. Metal and chloride percentages for compounds were conducted using a Shimadzu (AA) 680G atomic absorption spectrophotometer and potentiometric titration method on a 686-nitro processor-665 Dosimat-Metrom Swiss. A Eutech Instruments Cyberscan con 510 digital conductivity meter recorded molar conductance for complexes. Magnetic moments measurements were performed at 298.6 K using Sherwood Scientific Devised. The biological activities of compounds against bacterial and fungi species were examined at the Iraqi Ministry of Science and Technology's Central Service Laboratory.
Synthesis
Preparation of 2-(phenyl(2-phenylhydrazinyl)methyl)cyclohexan-1-one (M)
The synthesis of Mannich-base precursor 2-(phenyl(2-phenylhydrazinyl)- methyl)cyclohexan-1-one was achieved by adopting the reported method in 17-19 and as follows;
In to 100ml round-bottomed flask charged with a solution of CaCl2 (1.1g, 10mmol) in ethanol (10ml) and three drops of HCl (37%) were added benzaldehyde (1.06ml, 10mmol), phenylhydrazine (1.00ml, 10mmol) and cyclohexanone (1.03ml, 10mmol) consecutively. The reaction mixture was allowed to stir at room temperature for 12h, during which time an off-white solid was generated. This was removed by filtration, washed with distilled water (30ml) and ethanol (30ml), then dried in air. Yield: 1.8g (61%); m.p =158-160°C ; M.wt=294 amu. FT-IR (KBr) cm-1; 3471 ν(O-H)enol, 3414 and 3313 ν(N-H) stretching of the secondary amine, 1620 ν(C=C)w cm-1.
Synthesis of (E)-2-(2-(phenyl(2-phenylhydrazinyl)methyl)cyclohexylidene)hydrazine-1-carboxamide (HL)
A mixture of M (0.18g, 0.62 mmol) in 10 ml of EtOH was added with stirring semicarbazide hydrochloride (0.07g, 0.62 mmol) in 10 ml of EtOH. The reaction mixture was heated at reflux for 6h and then allowed to slow evaporation at RT. Upon standing, light-brown crystals were formed, which were collected by filtration, washed with cold ethanol (5ml) and diethyl ether (10ml) then dried in air. Yield: 0.12g (52%), m.p =179-180°C.; M.wt=351 amu. FT-IR (KBr) cm-1; 3460 ν(N4-H), 3309 ν(N5-H), 3286 ν(N2-H), 3194 and 3163 ν(N1-H), 1685 ν(C=O) 1647 cm-1 ν(C=N)imine. The 1HNMR (400 MHZ, DMSO-d6) ppm; δH= 10.26 (1H, s, N2-H), 7.84, 7.72-7.70 ppm (2H, dd, J= 1.2, 1.7 Hz), 7.08 (N4-H), 4.63 (N4-H), 3.05 (N1-H) and (C-H) of the cyclohexyl segment, 2.08 (C-H) ppm that is next to the (N4-H). EI–MS= m/z (%) = 305.10 observed for [M-(NH2CHO)]+ (100%), 278.0 [M-(NHCHO+N2)]+ (5%), 208.1 [M-(NHCHO+N2+(CH2)3N2)]+ (101%), 178.0 [M-(NHCHO+N2+(CH2)3N2+C2H4)]+ (72%), 134.1 [M-(NHCHO+N2+(CH2)3N2+C6H2)]+ (25%), 121.0 [M-(NHCHO+N2+(CH)11N)]+ (90%), 93.1 [M-(NHCHO+N2+(CH)11N+C2H6)]+ (45%), 77.1[M-(NHCHO+N2+ (CH)11N+C2H6+NH2)]+ (48%), 63.1[M-(NHCHO+N2 +(CH)11N+C3H6+NH2)]+ (28%) and 44.1 [M-(NHCHO+N2+(CH)11N+C5H12+NH2)]+ (33%) .
General Synthetic Procedure for Complexes
A solution of the semicarbazone ligand (0.1g, 0.28mmol) in ethanol (15ml) was allowed to stir then an ethanolic solution of potassium hydroxide was added dropwise to adjust the pH of the mixture to ca. 9. After stirring for 15 min an equivalent amount of the title metal chloride in 5ml of EtOH was added slowly. The mixture was heated at reflux with stirring for 3h. The resultant colored solid products formed were collected by filtration, washed with diethyl ether (5ml), and dried in air. Elemental analysis data, colors, and yields for the complexes are given in Table 1. The FT-IR and electronic data are listed in Tables 2 and 3, respectively.
Biological Evaluation
The semicarbazone ligand (HL) and its transition metal complexes were screened for their antibacterial activity against Escherichia coli, Pseudomonas auroginosa, Staphylococcus aureus and Bacillus subtilis strains. The activity was performed using the agar well diffusion method, in which wells were dug in the media using a sterile metallic borer with a center diameter of at least 6 mm 17,18. The activity of tested compounds was compared with Cefotaxime as an antibiotic standard. The tested compounds were dissolved in DMSO and incubated at 37°C for 24h. The role of DMSO against the tested species was also performed, which showed it has no effect. The concentrations of tested compounds and antibiotic standard were; 50mg/ml. The obtained data is included in Table 4. The antifungal activity of compounds was studied against two fungal strains Candida albicans and Rhizopus sporium species and data are placed in Table 4.
RESULTS and DISCUSSION
Chemistry
The formation of the Mannich-base precursor 2-(phenyl(2-phenylhydrazinyl)- methyl)cyclohexan-1-one (M) was accomplished through a one-pot three-component approach using calcium chloride as a catalyst.
The reaction of benzaldehyde, phenylhydrazine, cyclohexanone and CaCl2 in a 1:1:1:1 mole ratio, using EtOH as a medium, gave the title compound (Scheme 1). The condensation reaction of the precursor with the semicarbazide in a 1:1 mole ratio in EtOH medium resulted in the formation of the title ligand (E)-2-(2-(phenyl(2-phenylhydrazinyl)methyl)cyclohexylidene)hydrazine-1-carboxamide (HL), Scheme 1. The reaction of the ligand with metal chlorides of Cr(III), Mn (II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) in a 2:1 (L:M) mole ratio (using KOH as a base and EtOH as a medium) resulted in no pure complexes and subsequently, it was difficult to confirm the entity of the products. Therefore, the reaction proceeded in a 1:1 (L:M) mole ratio (using KOH as a base and EtOH as a medium), isolating six-coordinate and four-coordinate dimeric complexes, Scheme 2. KOH was essential to deprotonate the ligand and generate the monobasic species, and no reaction proceeded without adjusting the pH of the reaction to ca. 9. A range of physicochemical techniques characterized the prepared compounds (ligand and complexes). These include; 1H-NMR and mass spectroscopy (for ligand), micro-elemental analyses and metal and chloride ratio (Table 1), FT-IR (Table 2), and UV-Vis spectroscopy (Table 3). Conductance measurement was implemented to confirm metal complexes' electrolytic and non-electrolytic nature and to understand the number of counter ions out site the coordination sphere. The molar conductance of complexes in DMSO solutions indicated nonelectrolyte complexes' isolation. Further, the electronic data suggested the isolation of a six-coordinate dimeric Cr(III)-complex in which two water molecules require to give this structure. The FTIR data and the elemental microanalyses supported this. Based on the above data, dimeric complexes of the general formula [Cr(L)(Cl2)(H2O)]2 and [M(L)Cl]2 (where; M= Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II)) were isolated.
FT-IR data
FT-IR of precursor
The spectrum of M shows no band around 1700 cm-1 may relate to the carbonyl of the cyclohexanone (Figure 1). This could be attributed to a tautomerism phenomenon, the enol and keto-form 17-20. Therefore, the species in the solid state exists in the enol form. This was supported by the appearance of a band at 3471 cm-1 related to ν(O-H)enol, which was formed due to tautomerism within the cyclohexyl moiety, Scheme 3. This was backed up by a weak band at ca. 1620 cm-1 assigned to ν(C=C) of the cyclohexyl segment. The two bands at 3414 and 3313 cm-1 attributed to ν(N-H) stretching of the secondary amine. Peaks at 3055, 3024 and 1593 cm-1 in the M spectrum are attributed to the ν(C-H)aromatic, ν(C-H)aliphatic and ν(C=C)aromatic stretching 17-19,21, respectively.
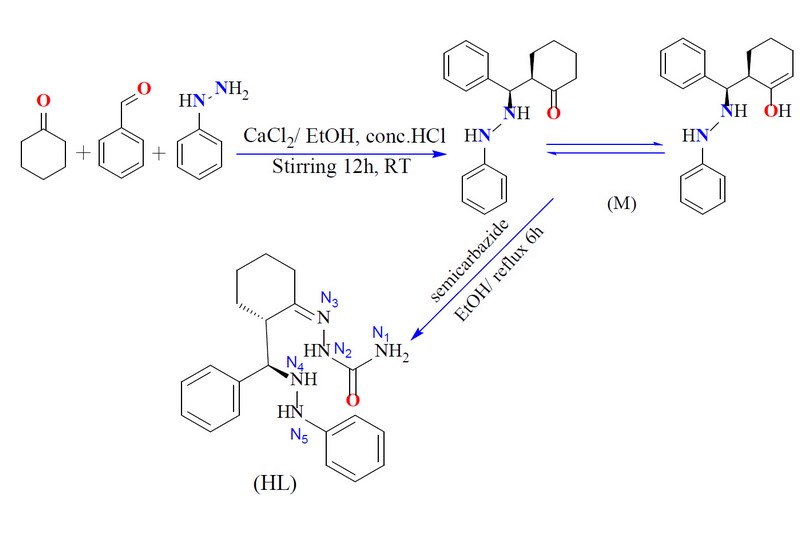
Figure 1. Synthesis route of the precursor and ligand.

Figure 2. Synthesis route of dimeric complexes.
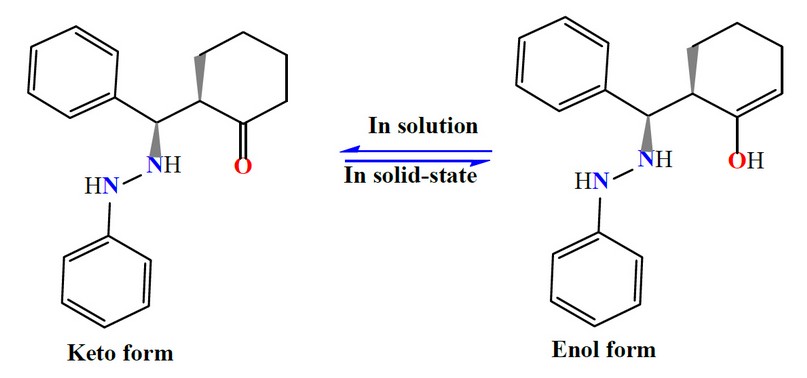
Figure 3. A tautomerism phenomenon within the cyclohexyl moiety.

Figure 4. FT-IR spectrum of M.
NMR, FT-IR and mass spectrum of semicarbazone ligand
The 1H-NMR of HL, Figure 2, which was recorded in DMSO-d6 indicated two sets of peaks in the aliphatic and aromatic regions. The singlet peak at 10.26 ppm (1H, s) corresponds to (N2-H). The signal observed at chemical shift 7.84 with a two proton integral and the doublet of doublet peak at 7.72-7.70 ppm (2H, dd, J= 1.2, 1.7 Hz) equivalent to two protons is correlated to the aromatic protons. A chemical shift that was recorded as a broad peak at 7.08 ppm correlated to (N5-H). The multiple signals appeared between 7.39-7.09 ppm, with integration corresponding to four protons assigned to the aromatic protons. Further, a peak at 6.46 ppm that is equivalent to two protons has related to the aromatic protons. The chemical shift observed at 4.63 ppm is assigned to (N4-H). A peak recorded at 3.05 ppm, which is equivalent to three protons is due to 2 protons of (N1-H) and one proton of the (C-H) of the cyclohexyl segment. A peak detected at 2.08 ppm equivalent to one proton was related to the (C-H) that is next to the (N4-H). Other peaks detected between 2.26 and 0.82 ppm correlated to the aliphatic protons of the remaining protons of the cyclohexyl segments.
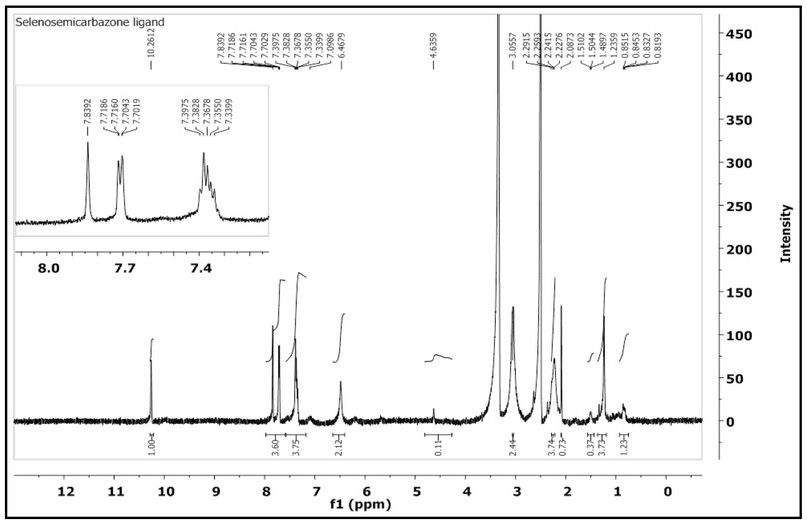
Figure 5. 1H-NMR spectrum of HL in DMSO-d6 solution.
The FT-IR of HL, Figure 5, showed bands at 3460 and 3309 cm-1 attributed to ν(N4-H) and ν(N5-H) of the hydrazine group, respectively. A band at 3286 cm-1 can be assigned to the ν(N2-H) stretching vibration. Peaks recorded at 3194 and 3163 cm-1 are attributed to the asymmetric and symmetric bands of ν(N1-H). The spectrum showed new bands at 1685 and 1647 cm-1, which correlated to the ν(C=O)amide group of semicarbazone and ν(C=N)imine 18, respectively.
The electrospray (+) mass spectrum of HL is placed in Figure, and the fragmentation pathway is in Scheme 4. The spectrum showed no peak at m/z = 351 amu may attribute to the molecular ion of the parent compound (M)+. Peaks detected at m/z=305.10 (100%), 278.0 (4.54%), 208.1 (10.61%), 178.0 (72.72%), 134.1 (25.75%), 121.0 (90.90%), 93.1 (45.62%), 77.1 (48.52%), 63.1 (28.55%) and 44.1 (33.33%) related to [M-(NH2CHO)]+, [M-(NHCHO+N2)]+, [M-(NHCHO+N2+(CH2)3N2)]+, [M-(NHCHO+N2+(CH2)3N2+C2H4)]+, [M-(NHCHO+N2+(CH2)3N2+C6H2)]+, [M-(NHCHO+N2+(CH)11N)]+, [M-(NHCHO+N2+(CH)11N+C2H6)]+, [M-(NHCHO+N2+ (CH)11N+C2H6+NH2)]+, [M-(NHCHO+N2+(CH)11N+C3H6+NH2)]+and [M-(NHCHO+N2+(CH)11N+C5H12+ NH2)]+, respectively.
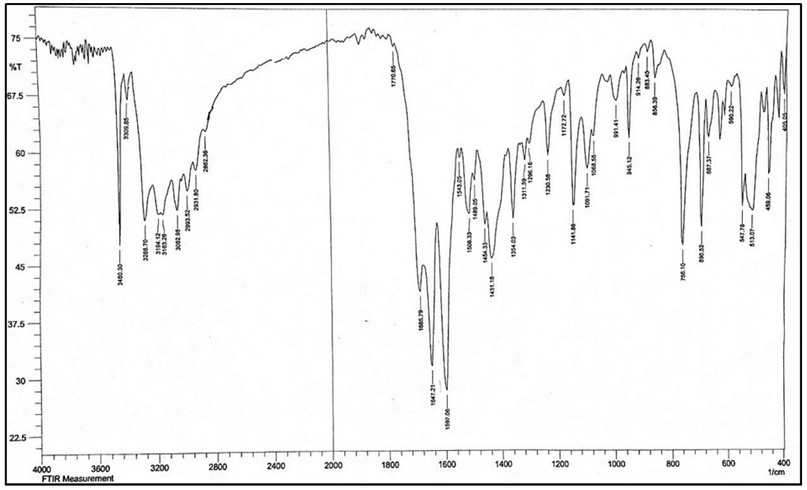
Figure 6. FT-IR spectrum of HL.
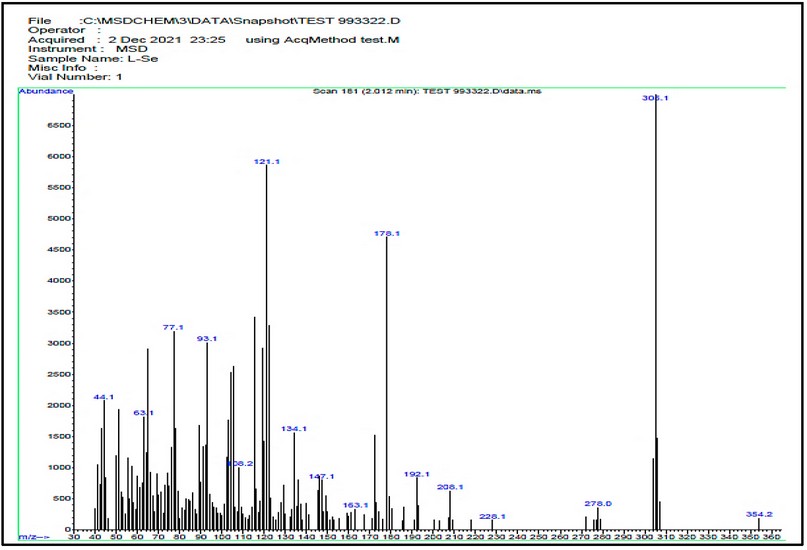
Figure 7. The electrospray (+) mass spectrum of HL.
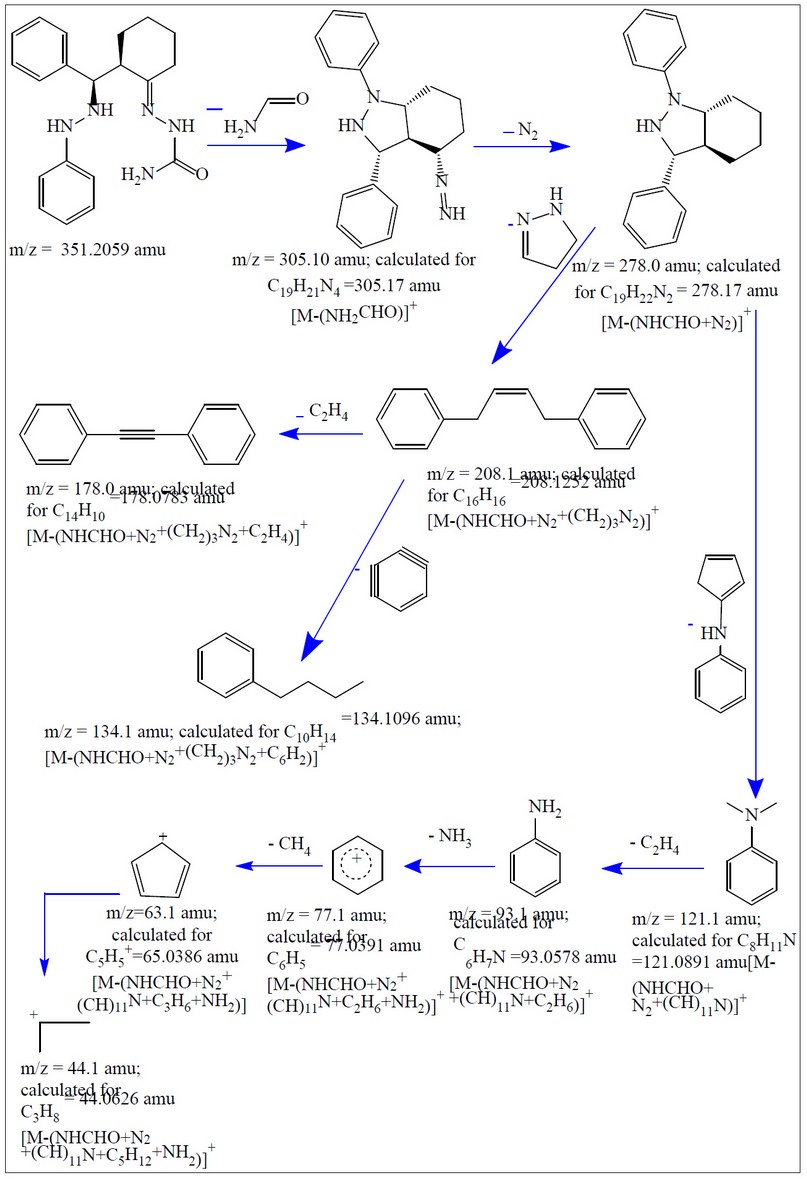
Figure 8. Fragmentation pathway of HL.
FT- IR spectra for complexes
The spectra of complexes showed no stretching band that may assign to ν(N2-H), indicating the deprotonation of the ligand upon complexation. The spectra revealed a shift in the imine stretching band, ν(C=N), by 4-20 cm-1 (compared with that at 1647 cm-1 in the free ligand) and appeared at 1631, 1631, 1627, 1639, 1643, 1635 and 1635 cm-1 in Cr(III), Mn(II), Co(II), Ni(II), Cu(II)), Zn(II) and Cd(II). respectively. The shifting of this band is due to the coordination of the nitrogen atom of the -C=N group of the imine to the metal center. The band at 1685 cm-1, which is due to ν(C=O)amide in the free ligand, is shifted by 11-39 cm-1 to lower frequency and appeared at 1666, 1664, 1672, 1668 and 1669 cm-1 in Cr(III), Ni(II), Cu(II)), Zn(II) and Cd(II) complexes, respectively. However, this band was shifted to a higher wavenumber (compared with the spectrum of the ligand) by 4 and 12 cm-1 and appeared at 1689 and 1697 cm-1 for complexes Mn(II)and Co(II), complexes respectively. This shifting is a piece of evidence for the coordination of the oxygen atom of the semicarbazone to the metal center 19. The spectrum of the free ligand revealed a peak at 3460 related to ν(N4-H) stretching of the hydrazine group. This peak suffered a shift, compared with the spectrum of the ligand, and appeared at 3433, 3402, 3440, 3444, 3375,3379 and 3394 for Cr(III), Mn(II), Co(II), Ni(II), Cu(II)), Zn(II) and Cd(II) complexes, respectively. The shift in the ν(N-H) confirmed the involvement of the nitrogen atom in the coordination with the metal ions 17. Bands displayed in the range 3302-3340 attributed to ν(N5-H). More, bands at 3199; 3159, 3190; 3159, 3175; 3109, 3159;3124, 3175; 3109, 3199;3170 and 3155; 3109 cm-1 assigned to ν(N1-H) in Cr(III), Mn(II), Co(II), Ni(II), Cu(II)), Zn(II) and Cd(II) complexes, respectively. The spectra indicated the appearance of new bands at 551, 567, 562, 536, 505, 543 and 543 cm-1 referred to ν(M-O) of Cr(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes, respectively [19,23]. Bands related to ν(M-N) were observed at 455, 451, 450, 420,462, 443 and 445 cm-1 for Cr(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II), respectively 17,18,22-24. Finally, The FT-IR spectra recorded bands related to ν(Cr_Cl) at 231;237 cm-1, ν(Mn_Cl) at 244 cm-1, ν(Co_Cl) at 243 cm-1, ν(Ni_Cl) at 247 cm-1, ν(Cu_Cl) at 239 cm-1, ν(Zn_Cl) at 246 cm-1, ν(Cd_Cl) at 248 cm-1 23-25. These bands confirmed the coordination of the chlorido moiety to the metal centre. Further, the appearance of two peaks in the spectrum of the Cr(III)-complex indicated the two coordinated chlorido moieties adopt the cis configuration. The spectrum of [Cr(L1)Cl2H2O]2.indicated peaks at 3525 and 694 cm-1 related to ν(OH) and ν(M-OH2), respectively 26.
Electronic spectra and magnetic moment measurements
The UV-Vis spectrum of HL exhibits peaks at 288, 343 and 363 nm due to π→π*, n→π* and charge transfer transitions 25-28, respectively. The electronic spectrum of the Cr(III)-complex indicated peaks at 462, 554 and 644 nm assigned to 4A2g(F)→4T2g(F), 4A2g(F)→4T1g(F) and 4A2g(F)→4T1g(P), respectively suggesting an octahedral geometry about the Cr atom 24,28. The spectrum of the Mn(II)-complex displayed peaks in the visible region at 664 and 730 nm attributed 6A1→4T2 (G) and 6A1→4A1 (G),4E(G), respectively. This data suggest the coordination sphere of the Mn atom is tetrahedral 29. The Co(II)-complex revealed peaks in the visible region at 503 and 620 nm correlated to 4T1(F)→4T1(p) and 4T1(F)→4A2(F) transitions, respectively and indicating tetrahedral structure around the Co atom 25. The spectrum of the Ni(II)-complex exhibited absorption peaks at 635, 797 and 840 nm attributed to 3T1(F)→ 3T1(P), 3T1(F)→ 3A2 (F) and 3T1(F)→ 3T2(F), respectively confirming tetrahedral arrangement about the Ni atom 29. Peaks recorded at 675 and 756 nm in the Cu(II)-complex spectrum were assigned to 2B1g→ 2B2g and 2B1g→ 2A2g, respectively, suggesting a distorted square planar geometry about the Cu(II). The spectra of the Zn(II) and Cd(II) complexes showed peaks around 281 nm assigned to the ligand field. Further, a peak at ca. 358 nm may attribute to charge transfer transition type M→L. As the two metal ions are d10 configuration, the spectra showed no bands in the d-d region 24,25,28,30. Therefore, the suggested tetrahedral geometry of the metal centre of the d10 configuration was based on the other characterisation tools. The magnetic susceptibility data for the HL complexes are presented in Table 3. The lowering of such magnetic moments may relate to the anti-ferromagnetic that occurred due to the formation of a dimeric species. The value for the Cr(III) complex is 3.87 BM, which indicates an octahedral geometry about the Cr atom. The magnetic moment measurements values of Mn(II), Co(II) and Ni(II) complexes are 2.46, 2.23 and 3.28 BM, respectively. These values are lower than the total spin-only values, indicating a tetrahedral geometry around the metal centre 31. The measured magnetic data of the Cu(II) complex 1.40 BM, may indicate a distorted square planer geometry about the Cu atom 31.

Table 1. Microanalysis and physical properties of HL and its complexes.

Table 2. FT-IR data (cm-1) of HL and its complexes.
Biological activity
The synthesised ligand and its metal complexes were tested for their microbiological activity against bacterial species: G-positive (Staphylococcus aurius, Bacillus subtilis) and G-negative (Escherichia coli, Pseudomonas auroginosa). The selected bacteria are considered to be the most harmful and deadly kind of bacteria. These bacteria are widely detected in the surgery operation rooms of hospitals. Further, two types of fungi were explored that have an impact on human beings (Candida albicans and Rhizopus sporium species).The commercial antibiotic Ceftriaxone was implemented as a reference drug for bacteria species 32. In general, all the prepared compounds showed excellent activity against bacterial strains, compared with the activity of Cefotaxime. Further, the Cu(II) and Ni(II)-complexes showed the highest activity against the two types of fungi, compared with the free ligand. The enhanced activity of the complexes against the examined microbial species may be attributed to the chelating theory 17-19,32. Further, the involvement of phenylhydrazine in the formation of the Mannich-base and subsequently in the structure of the ligand and complexes, could be another factor in enhancing the biological activity of compounds. Figures 5 and 6 and Table 4 display the biological activity data.

Table 3: UV-Vis, suggested structures and magnetic moment data of HL and its complexes.
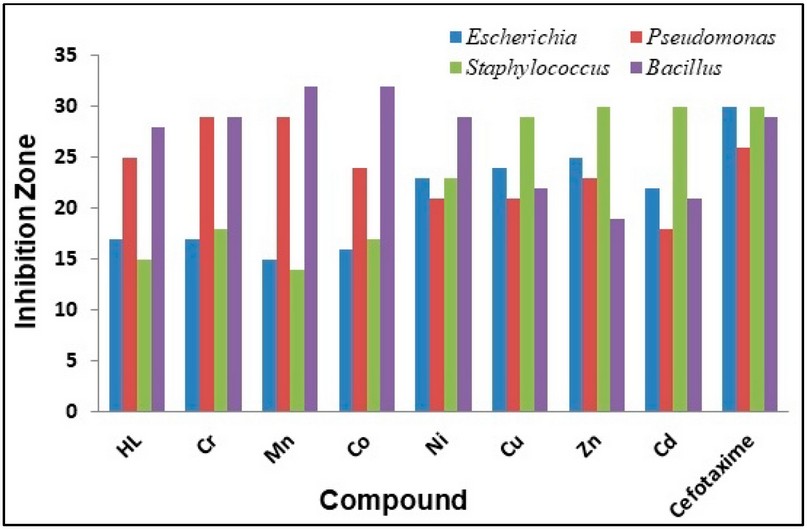
Figure 9. The inhibition zone diameter (mm) against bacterial species for HL ligand and its complexes.

Figure 10. The inhibition zone diameter (mm) against fungi for HL ligand and its complexes.

Table 4. The biological evaluation screening data (zone of inhibition in mm) of semicarbazone ligand and its complexes.
CONCLUSIONS
This work is based on the synthesis of a new semicarbazone Schiff-base ligand (E)-2-(phenyl(2-enylhydrazinyl)methyl)cyclohexylidene)hydrazine-1-carboxamide, and its dimeric complexes with Cr(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II). The formation of the ligand was derived from the reaction of the prepared Mannich-based precursor with the semicarbazide. The ligand is designed to include a phenylhydrazine segment within the structure of the Mannich base precursor, which is then used in the formation of the ligand. The objective of using phenylhydrazine in the formation of the ligand is to study the structural influence, coordination mode and biological effects that occurred on the ligand and its metal complexes. The entity of the prepared compounds was established using several analytical and spectroscopic techniques. These analyses indicated the Schiff base ligand behaves as a monobasic tridentate species and coordinated through the nitrogen atoms of the imine and hydrazine group and the oxygen carbonyl atom. Further, the isolation of a dimeric six-coordinate for the Cr(III)-complex and a dimeric four-coordinate for the Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) complexes was supported by the physicochemical investigation. Due to the formation of a dimeric species, these complexes' low magnetic moment values may account for their anti-ferromagnetic behavior. The biological activities of the prepared compounds against four types of bacteria and two types of fungi were investigated. It was found that compounds showed good anti-microbiological activity.
Author Contributions: Hadeel H. Abaas: Performed All Experiments, Data Curation, and manuscript Writing. Mohamad J. Al-Jeboori: Supervision, Methodology, Project Administration, Conceived the Experimental Plan, Analysed the Data, Writing- Original draft, Writing- Reviewing and Editing.
Acknowledgments: The authors thank the University of Baghdad, College of Education for Pure Science (Ibn Al-Haitham) and Department of Chemistry for providing Ms. HHA with the facilities for the MSc studentship and labs.
Conflicts of Interest: The authors declare there is no conflict of interest.
REFERENCES
1. Al-Rubaye, B. K.; Al-Jeboori, M. J.; Potgieter, H. Metal Complexes of Multidentate N2S2 Heterocyclic Schiff-base Ligands; Formation, Structural Characterisation and Biological Activity. Journal of Physics: Conference Series, 2021, 1879(22074), 3-20.
2. Al-Rubaye, B. K.; Brink, A.; Miller, G. J.; Potgieter, H.; Al-Jeboori, M. J. Crystal structure of (E)-4-benzylidene-6-phenyl-1,2,3,4,7,8,9,10-octahydrophenanthridine. Acta Crystallographica Section E: Crystallographic Communications, 2017, 73(7), 1092-1096.
3. Al-Rubaye, B. K.; Potgieter, H.; and Al-Jeboori, M. J. An Efficient One-Pot Approach for the Formation of Phenanthridine Derivative; Synthesis and Spectral Characterisation. Der Chemica Sinica, 2017, 8(3), 365-370.
4. Al-Jeboori, M. J.; Al-Jebouri, F. A.; and Al-Azzawi, M. A. Metal complexes of a new class of polydentate Mannich bases: Synthesis and spectroscopic characterisation. Inorganica Chimica Acta, 2017, 379(1), 163-170.
5. Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J. R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coordination Chemistry Reviews, 2018, 357, 144-172.
6. Naeimi, H.; Nazifi, Z. S.; Amininezhad, S. M.; Amouheidari, M. Synthesis, characterization and in vitro antimicrobial activity of some new Schiff bases and their complexes. The Journal of Antibiotics, 2013, 66(11), 687-689.
7. Mukherjee, T.; Pessoa, J. C.; Kumar, A.; Sarkar, A. R. Synthesis, structure, magnetic properties and biological activity of supramolecular copper (II) and nickel (II) complexes with a Schiff base ligand derived from vitamin B6. Dalton Transactions, 2013, 42(7), 2594-2607.
8. Fetoh, A.; Asla, K. A.; El-Sherif, A. A.; El-Didamony, H.; El-Reash, G. M. A. Synthesis, structural characterization, thermogravimetric, molecular modelling and biological studies of Co (II) and Ni (II) Schiff bases complexes. Journal of Molecular Structure, 2019, 1178, 524-537.
9. Al-Jeboori, M. J., Al-Dujaili, A. H., and Al-Janabi, A. E. Coordination of carbonyl oxygen in the complexes of polymeric N-crotonyl-2-hydroxyphenylazomethine. Transition Metal Chemistry, 2019, 34(1), 109-113.
10. Enyedy, É. A.; Bognár, G. M.; Nagy, N. V.; Jakusch, T.; Kiss, T.; Gambino, D. Solution speciation of potential anticancer metal complexes of salicylaldehyde semicarbazone and its bromo derivative. Polyhedron, 2014, 67, 242-252.
11. Li, L.; Zhang, Y. Z.; Liu, E.; Yang, C.; Golen, J. A.; Rheingold, A. L.; Zhang, G. Synthesis and structural characterization of zinc (II) and cobalt (II) complexes based on multidentate hydrazone ligands. Journal of Molecular Structure, 2016, 1110, 180-184.
12. Al-Rubaye, B. K.; Hussien, N. J.; Abul Rahman, A. A.; Yousif, E. I.; Al-Jeboori, M. J. "New organotin (IV) complexes derived from 3,4-dihydroxybenzaldehydeN(4)-ethyl-3-semicarbazone ligand; synthesis, characterisation and biological activity". Biochemical and Cellular Archives, 2020, 20(2), 6571-6579.
13. Hosseini-Yazdi, S. A.,; Samadzadeh-Aghdam, P.; Mirzaahmadi, A.; Khandar, A. A.; Mahmoudi, G.; Ruck, M.; Doert, T.; Balula, S. S.; Cunha-Silva, L. Synthesis, crystal structures, spectroscopic and electrochemical studies on Cu (II) and Ni (II) complexes with compartmental nitrogen–oxygen mixed donor ligands. Polyhedron, 2014, 80, 41-46.
14. Crook, A. J.; Lisic, E. C.; and Ensor, D. D. Thiosemicarbazone and semicarbazone chelating resins and their potential use in environmental applications. Separation Science and Technology, 2012, 47(14-15), 2225-2229.
15. Asghari, A.; Ghazaghi, M.; Rajabi, M.; Behzad, M.; Ghaedi, M. Ionic liquid-based dispersive liquid-liquid microextraction combined with high-performance liquid chromatography-UV detection for simultaneous preconcentration and determination of Ni, Co, Cu and Zn in water samples. Journal of the Serbian Chemical Society, 2014, 79(1), 63-76.
16. Liaqat, M.; Mahmud, T.; Ashraf, M.; Muddassar, M.; Imran, M.; Ahmad, T.; Mitu, L. Synthesis, Characterization and Biological Activities of a Novel Mannich Base 2-[(3,4-dimethoxyphenyl)(pyrrolidinyl)methyl] cyclohexanone and its Complexes with Cu (II), Ni (II), Co (II) and Fe (II) Ions. Revista De Chimie, 2017, 68(12), 2845-2849.
17. Al-Qazzaz, A. H.; Al-Jeboori, M. J. New metal complexes derived from Mannich ligands; synthesis, spectral investigation and biological activity. Biochemical and Cellular Archives, 2020, 20, Supplement 2, 4207-4216.
18. Hussain, S. A.; Al-Jeboori, M. J. New Metal Complexes Derived from Mannich-Base Ligand; Synthesis, Spectral Characterisation and Biological Activity. Journal of Global Pharma Technology, 2019, 11(2), 548-560.
19. Saleh, H. A.; Al-Khatib, A. S.; and Al-Jeboori, M. J. Synthesis, structural characterisation and biological evaluation of new semicarbazone metal complexes derived from Mannich-base. Biochem. Cell. Arch., 2021, 21(1), 159-168.
20. Moradi, R.; Jameh-Bozorghi, S.; Kadivar, R.; Mahdiani, A.; Soleymanabadi, H. Study of mechanism keto-enol tautomerism (isomeric reaction) structure cyclohexanone by using Ab initio molecular orbital and density functional theory (DFT) method with NBO Analysis. APCBEE Procedia, 2012, 3, 70-74.
21. Al-Jeboori, M. J.; Al-Fahdawi, M. S.; and Sameh, A. A. New homoleptic metal complexes of Schiff bases derived from 2,4-di-p-tolyl-3-azabicyclo [3.3.1] nonan-9-one. Journal of Coordination Chemistry, 2009, 62(23), 3853-3863.
22. Al-Jeboori, M. J.; Ameer, A. A.; Aboud, N. A. (2016). Novel Pentadentate Ligand of N3S2 Donor Atoms and It's Complexes With (NiII, CoII, CuII, FeII, HgII, AgI and ReV. Journal of Ibn Al-Haitham for Pure and Applied Sciences, 2004, 17(3),80-89
23. Al-Jeboori, F. H. A.; Hammud, K. K.; Al-Jeboori, M. J. Synthesis and characterization of new acyclic octadentate ligand and its complexes. Iranian Journal for Science & Technology, 2014, 38A (4): 489-497.
24. Al-Jeboori, M. J.; Abdul-Ghani, A. J.; Al-Karawi, A. J. Synthesis and structural studies of new Mannich base ligands and their metal complexes. Transition Metal Chemistry, 2008, 33(7), 925-930.
25. Ahmed, R. M.; Yousif, E. I.; Al-Jeboori, M. J. Co(II) and Cd(II) complexes derived from heterocyclic Schiff-bases: synthesis, structural characterisation, and biological activity. The Scientific World Journal, 2013, 2013, 1-6.
26. Al-Jeboori, M. J.; Hasan, H. A.; Al-Sa'idy, W. A. J. Formation of polymeric chain assemblies of transition metal complexes with a multidentate Schiff-base. Transition Metal Chemistry, 2009, 34(6), 593-598.
27. Mawat, T. H.; Al-Jeboori, M. J. Novel Metal Complexes Derived from Selenosemicarbazone Ligand; Synthesis, Spectral Investigation and Biological Activity. J. Global Pharma Tech, 2019, 11(09), 126-138.
28. Abdul-Ghani, A. J.; Al-Jeboori M. J.; Al-Karawi, A. M. Synthesis and characterisation of new N2S2 and N2O2 Mannich base ligands derived from phosphinic acid and their metal complexes. Journal of Coordination Chemistry, 2009, 62(16), 2736-2744.
29. Shaker, S. A.; Khaledi, H.; Cheah, S. C.; and Ali, H. M. New Mn (II), Ni (II), Cd (II), Pb (II) complexes with 2-methylbenzimidazole and other ligands. Synthesis, spectroscopic characterization, crystal structure, magnetic susceptibility and biological activity studies. Arabian Journal of Chemistry, 2016, 9, S1943-S1950.
30. Mawat, T. H.; Al-Jeboori, M. J. Synthesis, characterisation, thermal properties and biological activity of coordination compounds of novel selenosemicarbazone ligands. Journal of Molecular Structure, 2020, 1208, 127867.
31. Ahmed, R. M.; Hamdan, T. A.; Numan, A. T.; Al-Jeboori, M. J.; Potgieter, H. Formation of polymeric assemblies of six-coordinate metal complexes with mixed bridges of dicarboxylato-azido moieties. Complex Metals: An Open Access Journal, 2014, 1(1), 38-45.
32. Etheb, A. M.; Al-Jeboori, M. J. Synthesis, Structural Characterisation and Biological Activity of New Mannich Compounds Derived from Cyclohexanone Moiety, HIV Nursing, 2022, 22 (2), 3659-3666.
Received: 2 January 2023/ Accepted: 19 April 2023 / Published:15 June 2023
Citation: Abaas H J, Al-Jeboori M J. New dimeric complexes with semicarbazone mannish-based ligand; formation, structural investigation and biological activity. Revis Bionatura 2023;8 (2) 15. http://dx.doi.org/10.21931/RB/2023.08.02.15
