2023.08.02.7
Files > Volume 8 > Vol 8 No 2 2023
Antifungal activity of metabolites from Trichoderma spp. against Fusarium oxysporum
González M.F 1,2,3* Galarza L 1,2 Valdez L.L 3 Quizhpe G.M 3
1 Escuela Superior Politécnica del Litoral, ESPOL, Biotechnology Research Center (CIBE), Campus Gustavo Galindo, Km. 30.5 vía Perimetral, P.O. Box 09-01-5863, Guayaquil, Ecuador.; [email protected] .
2 Escuela Superior Politécnica del Litoral, ESPOL, Faculty of Life Sciences, Campus Gustavo Galindo, Km. 30.5 vía Perimetral, P.O. Box 09-01-5863, Guayaquil, Ecuador; [email protected].
3 Universidad de Guayaquil, Facultad de Ingeniería Química, Facultad de Ciencias Química Cdla. Salvador Allende, Av. Delta entre Av. Kennedy, P.O. Box 471, Guayaquil, Ecuador.
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.7
ABSTRACT
The Trichoderma genus is well known as one of the most valuable biological control agents against several phytopathogens used in different plant species. Managing phytopathogenic fungi using the Trichoderma genus through various associated antifungal mechanisms is a sustainable and eco-friendly strategy that reduces the harmful presence of pathogens in soil, roots and aerial parts of plants. However, using biocontrol agents combined with chemical pesticides has evidenced further potential to reduce pathogen growth and benefit plant development. A better characterization of active metabolites secreted by Trichoderma and their mechanisms of action is necessary to improve its use as a biocontrol agent. This review summarizes current evidence on Trichoderma spp., used as a biocontrol against Fusarium oxysporum, the active secondary metabolites secreted by the former fungi, and the effect of three widely used agrochemicals to control the latter, namely Mancozeb, Chlorothalonil, and Propiconazole. A total of 155 studies were selected and used to extract information that was analyzed, resulting in more than 590 identified secondary metabolites. Fifty-four percent of these have at least one biological function. Results highlight the potential of T. harzianum and T. reesei as biological control agents to control Fusarium oxysporum. The antifungal activity of T. Espirale is associated with enzymatic reactions. Additional findings show that management of diseases caused by F. oxysporum can be combined by using Trichoderma as biological control and agrochemicals to reach: (1) higher access to the different plant tissues; (2) higher degradation of the cell wall; and (3) and activation of oxidative metabolism of Trichoderma.
Keywords: Trichoderma, secondary metabolites, fungicide, mycoparasitism, biocontrol, Fusarium oxysporum
INTRODUCTION
Fusarium oxysporum is one the most economically important phytopathogens when referring to agriculturally important crops such as bananas and other crops 1. The fungus infects the host plant through the roots or stems, causing wilt, blight, rot, and cancer of many plant species leading to significant yield loss in economically important crops such as banana 2, onion 3, tomato 4, chili 5, watermelon 6, cabbage 7, ginger 8, chickpea 9, soybean 10,11, eggplant plants 12, as well as ornamental plants like Chrysanthemum spp., Dianthus spp., Gerbera spp., Gladiolus spp. and Lilium spp. 1. No curative control method is currently available against this pathogen 1. Current approaches to control F. oxysporum infestation are based on prophylactic measures and cultural practices 1 like keeping tools, soils and substrates in good sanitary conditions, planting resistant or tolerant genotypes, paying particular attention to crop monitoring, appropriate management of irrigation and crop rotation 1.
Chemical fungicides have also been essential in managing Fusarium oxysporum wilt for decades. However, these chemical control agents often become ineffective since pathogens may develop resistance, and the chemicals may adversely affect soil fertility and accumulate in the crop 13. Since chemical pesticides are not selective, they can influence many beneficial non-target biotas and potentially harm the farmer's health 14–16. Due to the harmful effects of these products, different policies that limit pesticide use have been implemented in several nations in the world 1,17.
Alternatively, biocontrol agents aim to regulate the growth of the pathogen with less harm to the plant and farmers. In this line, mycopesticides are exciting products because they use several mechanisms of action that reduce plant disease caused by phytopathogenic fungi 1,13,18. Biopesticides are often less toxic than chemical products and decompose quickly. This can avoid pollution problems, resistance and residue concerns. Biopesticides generally affect only the target pest and closely related organisms, thereby protecting other organisms living in the same environment. The commercial evolution of the biopesticide market is promising to be a potential tool for pathogen control with a current annual growth rate of 14.1% 13.
Trichoderma spp. comprises more than 200 validly described species distributed in soils worldwide and across various habitats and are considered a valuable resource for structurally novel natural products with diverse bioactivities, including biological control of phytopathogens 19. In the interest of obtaining more effective methods of pathogen control, plant growth-promoting rhizosphere microorganisms have been used as a consortium or in combination with chemical pesticides by our group and other authors 20–23. Bioassays have revealed great potential to improve current methods of managing antifungal treatments to plant cultivars. Yet, summarized evidence about active metabolites and the mechanism of actions of both biocontrol agents and chemical pesticides is needed to better use this possibility.
In this work, we reviewed the use of Trichoderma spp. as a biocontrol agent and the secondary metabolites and enzymes that have been characterized as active molecules. As additional findings, we also summarized the antifungal activity of commercial fungicides Mancozeb, Chlorothalonil, and Propiconazole, which are often used to control plant diseases caused by F. oxysporum.
Narrative Findings on Trichoderma spp
Trichoderma is a genus that belongs to the family Hypocreaceae and comprises many different fungi strains found in most diverse ecosystems 19. Trichoderma strains proliferate and have a characteristic morphology, white and cottons at the beginning, then developing into yellowish green to deep green compact tufts. Trichoderma strains are characteristically branched 24. The distinctive species categorized in the genus of Trichoderma are hard to differentiate morphologically. Based on morphology, Trichoderma strains have been classified into five sections: Saturnisporum, Pachybasium, Longibrahiatum, Trichoderma and Hypocreanum 25. New genetic tools and physiological activity are used to determine the different functional groups within Trichoderma spp. Thus, current identification methods of Trichoderma strains include morphological and molecular characterization.
Many Trichoderma fungi act as biocontrol agents of phytopathogens and plant growth promoters 26. They can also stimulate plant defense mechanisms against insect pests and be efficient soil bioremediation agents 27. Trichoderma spp. can also be used in waste/organic materials decomposition and polluted area detoxification 28. Some examples have emerged as human pathogens, for example T. longibrachiatum. Consequently, while the studies on effective biocontrol fungal are ongoing, further research to avoid the risk for humans, plants, and other organisms contributed by Trichoderma spp. also need to be accomplished.
The Mechanisms of biological control by Trichoderma spp
Biological control by Trichoderma spp. is based on the activation of indirect and direct mechanisms. Direct and indirect mechanisms can act synergistically and depending on species and strain 29. The indirect mechanisms are competition for space and nutrients, growth promotion, and systemic resistance induction. The mechanisms by which Trichoderma induces systemic resistance in plants vary depending on plant species, Trichoderma species, pathogen species, abiotic stress conditions, and culture methods. It has been shown that Trichoderma colonization of plant rhizosphere may simultaneously activate both systemic acquired resistance and induced systemic resistance mechanisms of the plant. Trichoderma is also known to induce the resistance of plants towards diseases by root architecture alteration during the interaction with pathogens 30.
Direct mechanisms are mycoparasitism and the production of active metabolites and lytic enzymes 3,31. Mycoparasitism, the ability to parasitize on fungi, is a unique characteristic of Trichoderma since they can parasitize even taxonomically close species 19. The antifungal activity of Trichoderma against phytopathogenic fungi is attributed to the combined action of secondary metabolites (SMs) and hydrolytic enzymes i.e., cellulases, proteases, chitinases, and xylanases 3,32,33. About 500,000 secondary metabolites have been described; of these, 15.600 (47 %) are of fungal origin.
Characterization of genes involved in fungal–fungal interactions has indicated that are mainly those involved in signal transduction, fungal cell wall degradation, and production of secondary antifungal metabolites (SMs) 19.
Secondary metabolites and enzimes produced by Trichoderma spp
SMs are not essential for normal growth but are synthetized for specific environmental conditions. SMs can be either volatile or non-volatile organic compounds. Volatile SMs diffuse over a distance through systems in the soil affecting the physiology of competitor organisms 34–36. Non-volatile SMs exert their activity through direct interactions between Trichoderma species and their antagonists 35.
Our search for current evidence on SMs secreted by Trichoderma spp. Or enzymes resulted in annotating 590 unique compounds listed in the sup[A5] plementary Table S1. It includes many structural classes like pyrones, butenolides, steroids, peptaibols and terpenoids 19. Fifty-four percent of all SMs or enzymes retrieved in our search have at least one biological effect associated, described in Table S1. Even though this list of biological activities should not be considered exhaustive, it allows appreciation of the incredibly broad range of biological activities of Trichoderma SMs i.e, antifungal, antibacterial, antitumor, DPPH-radical-scavenging, positive effect on plant growth and development, among others (Table S1). Further investigation is required using isolated compounds to obtain a comprehensive understanding of all effects at different for the different combinations.
The list of SMs shown in supplementary Table S1 is consistent with previous papers that emphasize that the quality and the number of volatile compounds produced are variable for each strain of Trichoderma. As example of the diversity in SMs produced by different Trichoderma species. A total of 115 SMs were reported for T. reesei, T. harzianum and T. spirale (Table S1). SM or enzymes identified for T. reesei, T. harzianum and T. spirale are indicated in Table 1, 2 and 3, respectively, which could be potentially used to control Fusarium oxysporum, due to their antifungal activity.
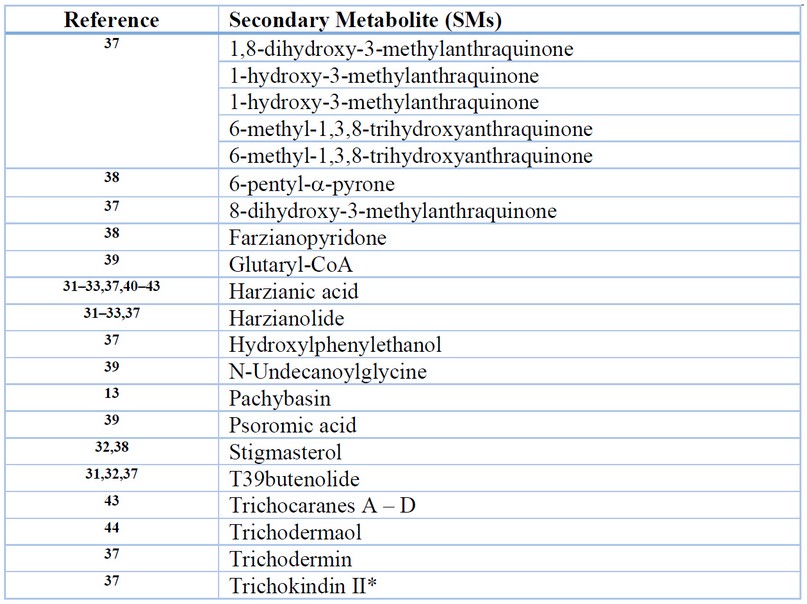
Table 1. SMs of Trichoderma harzianum with antigungal activity
This result should not be understood as only T. harzianum secretes all these compounds. Genes encoding for proteins responsible for synthesizing these SMs are usually not expressed constitutively but due to interactions with the pathogen in the plant rhizosphere 4,45. For example, the SM trichosetin, presumably secreted by T. harzianum, has only been identified in dual culture of T. harzianum and calli of Catharathus roseus but not in single cultures 31. However, the vast diversity of SMs isolated and characterized from T. harzianum indicates the great potential value of this fungus as a biocontrol agent against phytopathogenic fungi.
In vitro and in vivo assays have shown T. harzianum isolates with higher inhibitory activity against F. oxysporum (F3) than other Trichoderma species 46. Biocontrol potential of T. harzianum against Fusarium Oxysporum has been demonstrated in vitro e in vivo against F. oxysporum in Poplar 47, ginger 8, cucumber 29,48, lettuce 49, white yam 50, chili 51, tomato and cucumber 52. Nonetheless, T. reesei is one of the top fungal species used in industrial biotechnology and is used safely for decades in enzyme production. In contrast to T. harzianum, T. reesei is considered to have a limited production of mycotoxins 53. Table 2 lists all SMS associated with antifungal activity.
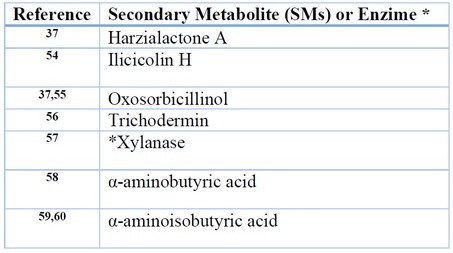
Table 2. SMs or enzymes of Trichoderma reesei with antifungal activity
Despite the literature did not specifically listed the SMs of T. spirale associated with the antifungal activity, it was worth to list the asociated enzimes Trichoderma spirale (Table 3). This list is short but shows the potential use of T. spirale [A9] in the control of pathogenic fungi.
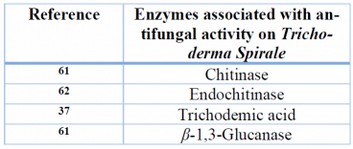
Table 3. Enzymes associated with antifungal activity of Trichoderma spirale
In this work, we reviewed the use of Trichoderma spp. as biocontrol agents, the secondary metabolites characterized as active molecules and the enzymes found in this bibliographic review which present antifungal characteristics that act directly on the phytopathogenic fungi. The following graph highlights the following strains of Trichoderma spp., T. reesei, T. harzianum and T. spirale with the most critical metabolites and enzymes
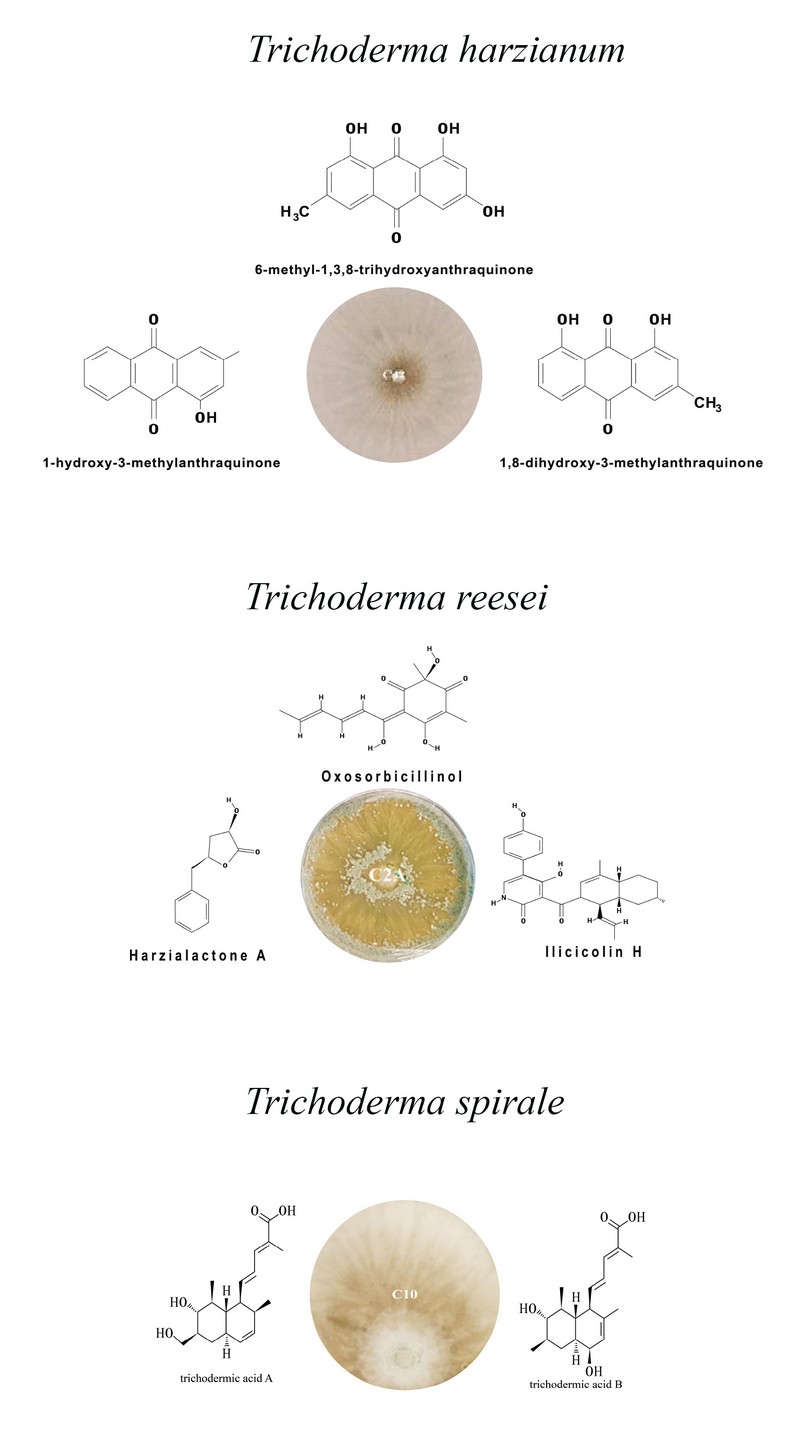
Figure 1. Secondary metabolites and enzymes associated with antifungal activity that stand out in T. reesei, T. harzianum and T. spirale strains identified in the literature review on using Trichoderma spp. as biocontrol agents against Fusarium Oxysporum.
Additional findings on the use of Trichoderma spp. and 3 synthetic pesticides[A10]
The low input cost and higher crop productivity of applying biological control agents (or biopesticides) are the economic benefits observed when compared to synthetic pesticides 63. Thus, the use of Trichoderma is regarded as a sustainable approach not only ecologically but also from an economic perspective.
However, using microbial-based products as biocontrols or biostimulants has some disadvantages compared to their chemical counterparts. Microbial products have a limited shelf life and require special conditions for conservation to maintain viability and efficacy 44. Also, they have constraints due to dependency on the crop, geographical, and meteorological regimes and pathogens 3,63.
One interesting approach that has emerged to cope with the advantages and limitations of the different methods to control crop infestation with F. oxysporum is the simultaneous application of Trichoderma as biological control with chemical pesticides and other biological control agents. For example, a combined treatment with T. polysporum LCB50 and irrigation with liquid compost applied resulted in a strong synergistic effect in controlling melon wilt and a 100% increase in the productivity of commercial fruit 64.
Recent results from our group have shown a synergistic effect using T. reesei and Mancozeb, inhibiting the mycelial growth of F. oxysporum (F1)20. Also, a synergic activity was obtained in vitro assays using T. ressei combined with Chlorothalonil or Propiconazole (unpublished data). However, the molecular basis of these agents' biological activity that results in an increased capacity to inhibit F. oxysporum infection is unknown.
Chlorothalonil (tetrachloroisophthalonitrile) and Mancozeb (manganese ethylene bis (dithiocarbamate) (polymeric) complex with zinc salt) are multisite enzyme inhibitors that act as protective broad spectrum fungicides 65. Both are non-systemic, preventive fungicides that form a protectant barrier at the surface of the plant against the germination of spores and inhibit pathogen development 65,66. Propiconazole (((2RS 4RS;2RS,4SR)-1-[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-ylmethyl]-1H-1,2,4-triazole)) belongs to a group of systemic fungicides that destabilize the cell membrane integrity and affects ergosterol biosynthesis through the inhibition of C14-demethylation 65. Systemic fungicides are adsorbed into the leafs and translocated via the xylem, thus protecting the plant and controlling the circulating pathogens 65.
The co-inoculation strategy is possible as long as the fungus used as biopesticide tolerates the minimum concentration of the chemical fungicide required. This latter does not interfere with its development but contributes to increased control over the phytopathogenic specie. In this scenario, as proposed by Pelàez-Alvarez et al. (2016) 23, the presence of the chemical pesticide retards the growth of the phytopathogen, providing an advantage in the competition for space and nutrients in favor of the biopesticide. Chemical sensing of the competing fungus would induce the secretion of an arsenal of SMs that may act in both senses, facilitating the activity of the chemical fungicide and stimulating the plant's defense system 29,67. Hence, the movement of chemical fungicides takes place from the upper parts of the plant and, in the case of those of systemic activity, disseminates to lower parts.
F. oxysporum fungus enters through the roots and disseminates throughout the plant using the vascular system. In contrast to chemical fungicides, Trichoderma fungi are part of the rhizosphere and generally grow on plant root surfaces and therefore control root diseases in particular 68. Consequently, using Trichoderma as a biological control agent will provide an effective first barrier at the site of infection that will be complemented by the activity from top to bottom of chemical pesticides.
Previous work on Trichoderma spp. used as biocontrol agents have shown that cell wall degrading enzyme secreted by fungi i.e chitinase, cellulase, protease, and β-(1-3) glucanase and peptaibols are produced concurrently during biocontrol and interact synergistically as antifungal agents 69. The proposed mechanism for such an effect is based on the fact that enzymes degrade the cell wall of host fungal pathogens. This activity directly inhibits the growth of the pathogen, at the same time, facilitates the access of peptaibols to the cellular membrane. Peptaibols. are small peptides of 15-20 residues characterized by non-standard amino acids in their sequences, with a special propensity for aminoisobutyric acid. The antimicrobial activity of peptaibols is related to their capacity to form pores in lipid membranes 70.
The same synergistic effect has been described for the activity of cell wall degrading enzymes and other SMs targeting specific target molecules in inhibiting F. oxysporum by T. asperellum 29. Table 4 summarizes SMs identified as present in extracts with potent antifungal activity against F. oxysporum; or assayed from purified preparations and with proven inhibitory activity against this phytopathogen. Yet, important differences have been reported in the activity of cell wall degrading enzymes for T. asperellum and T. harzianum 29. Thus, this mechanism could not be similarly effective for all Trichoderma species.
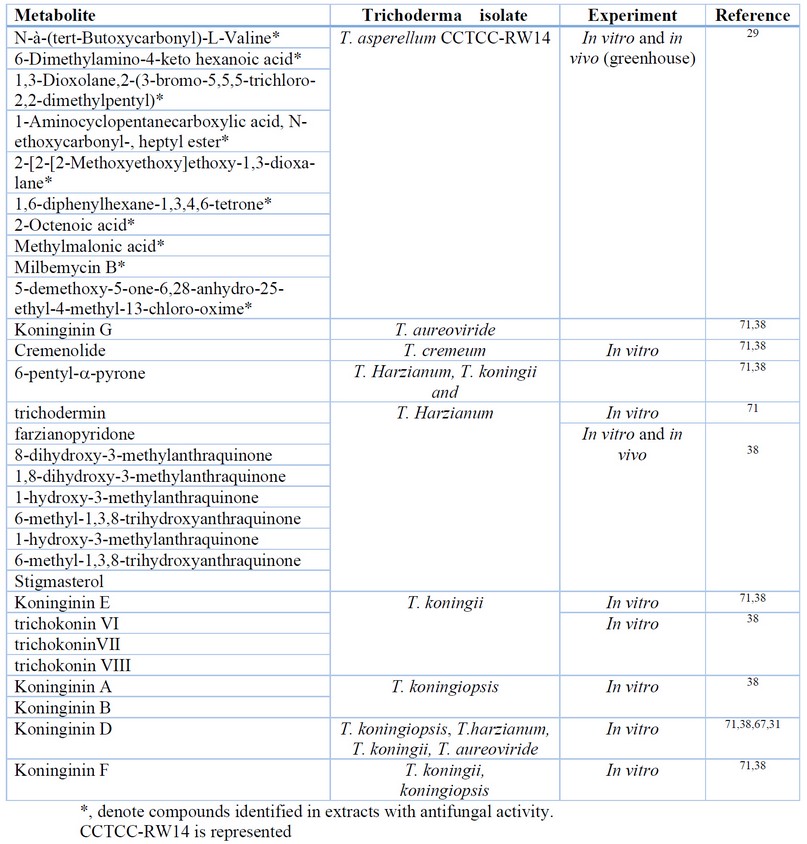
Table 4. Metabolites identified in extracts, or purified, with antifungal activity against Fusarium oxysporum.
A similar synergistic activity could explain the outcome observed by co-inoculating Trichoderma species with biocontrol capacity against F. oxysporum and Mancozeb, Chlorothalonil and Propiconazole 20.. It is reasonable to expect a similar effect on facilitating the penetration of these chemical fungicides.
Systemic effects resulting from Trichoderma interaction with the plant would also contribute to the observed synergistic effect when used with chemical pesticides. Reactive oxygen species scavenging enzymes have been found significantly increased in plants treated with Trichoderma T-soybean, T. longibrachiatum and T. harzianum, thus improving plant resistance to oxidative stress 3,11,72. Exposure of plants to pesticides has evidenced that most of these chemicals lead to the development of oxidative stress 17. Also, root colonization by Trichoderma has been found to result in intensified levels of defense-related, including β-peroxidases and hydroxide lyase of lipoxygenase-pathway of the plant 73. Moreover, it has been evidenced that T. harzianum alleviates oxidative stress by minimizing reactive oxygen species accumulation during F. oxysporum infection 52. Thus, the contribution to activating a systemic response to oxidative stress in plants could be another level of cooperative action between chemical and biological control agents to control the attack of this phytopathogenic fungus.
CONCLUSIONS
Deleterious effects caused by F. oxysporum on plant species cause significant economic losses in agriculture at domestic and industrial levels. This review presents an organized narrative of information starting with the antifungal mechanism of Trichoderma, listing the SMs and enzymes involved in these mechanisms and finally the potential synergy of 3 synthetic pesticides for a better control of F. oxysporum. Our findings suggest that there is a need to develop more effective and ecologically friendly methods of controlling F. oxysporum, compared to the current control methods. Both chemical and biological control agents have individually played important roles protecting crops for millenniums. Also, both have advantages and disadvantages in their use. Thus, recent approaches have proposed the simultaneous use of chemical and biological pesticides and obtained promising results evidencing a synergistic activity controlling F. oxysporum infestation at in vitro and in vivo experiments. A better understanding of modes of action and cooperative effects of these two types of fungicide agents should let make better use of them in co-inoculation programs. The review of current knowledge on modes of action of Trichoderma in the control of F. oxysporum infection as well as the chemical fungicides Mancozeb, Chlorothalonil and Propiconazole confirms that their inhibitory activities may be compensatory and may lead to synergistic effects.
Supplementary Materials: Supplementary Table 1 (S1) is available under request.
Author Contributions: GMF, GL, VL and QG made substantial contributions conception and design, or acquisition of data, or analysis and interpretation of data. GMF and GL contributed drafting the article or revising it critically for important intellectual content. GMF and GL revised the final version of the manuscript before publication. GMF and GL ensures that any part of the work was appropriately investigated and resolved.
Funding: This study did not receive any funding
Institutional Review Board Statement: Ethical review and approval were waived for this study because it does not involve humans or animals.
Data Availability Statement: Data is available fully in open access.
Acknowledgments: Thanks to the scientific advisors who guided the initial design of this review.
Conflicts of Interest “The authors declare no conflict of interest.”
REFERENCES
1. Lecomte C, Alabouvette C, Edel-Hermann V, Robert F, Steinberg C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol Control. 2016;101:17-30. doi:10.1016/j.biocontrol.2016.06.004
2. Damodaran T, Rajan S, Manoharan M, Gopal R. Biological Management of Banana Fusarium Wilt Caused by Fusarium oxysporum f . sp . cubense Tropical Race 4 Using Antagonistic Fungal Isolate CSR-T-3 ( Trichoderma reesei ). 2020;11(December):1-19. doi:10.3389/fmicb.2020.595845
3. Abdelrahman M, Abdel-Motaal F, El-Sayed M, et al. Dissection of Trichoderma longibrachiatum-induced defense in onion (Allium cepa L.) against Fusarium oxysporum f. sp. cepa by target metabolite profiling. Plant Sci. 2016;246:128-138. doi:10.1016/j.plantsci.2016.02.008
4. Cucu MA, Gilardi G, Pugliese M, Lodovica M, Garibaldi A. AGROINNOVA - Centre of Competence for the Innovation in the Agro-Environmental Sector , Department of Agricultural , Forest and Food Sciences ( DISAFA ), Turin University , Largo P . Biol Control. Published online 2019:104158. doi:10.1016/j.biocontrol.2019.104158
5. Utami U, Nisa C, Putri AY, Rahmawati E. The potency of secondary metabolites endophytic fungi Trichoderma sp as biocontrol of Colletotrichum sp and Fusarium oxysporum causing disease in chili The Potency of Secondary Metabolites Endophytic Fungi Trichoderma sp as Biocontrol of Colletotrichum sp. 2019;080020.
6. Petkar A, Harris-Shultz K, Wang H, Brewer MT, Sumabat L, Ji P. Genetic and phenotypic diversity of Fusarium oxysporum f. Sp. Niveum populations from watermelon in the southeastern United States. PLoS One. 2019;14(7). doi:10.1371/journal.pone.0219821
7. Palyzová A, Sokolová L. Metabolic profiling of Fusarium oxysporum f . sp . conglutinans race 2 in dual cultures with biocontrol agents Bacillus amyloliquefaciens , Pseudomonas aeruginosa , and Trichoderma harzianum. Published online 2019:779-787.
8. Das MM, Haridas M, Sabu A. Biological control of black pepper and ginger pathogens, Fusarium oxysporum, Rhizoctonia solani and Phytophthora capsici, using Trichoderma spp. Biocatal Agric Biotechnol. 2018;3. doi:10.1016/j.bcab.2018.11.021
9. Faisal M, Tiwari S, Tiwari U. Variation in antagonistic effects of Trichoderma species on Fusarium oxysporum f. sp. ciceri. ~ 1828 ~ J Pharmacogn Phytochem. 2019;8(5):1828-1832. http://www.phytojournal.com
10. Cruz DR, Ellis ML, S. GPM, L. F. S. Leandro. Isolate × Cultivar Interactions, In-Vitro Growth and Fungicide Sensitivity of Fusarium oxysporum Isolates Causing Seedling Disease on Soybean. Plant Dis. Published online 2018:1-38.
11. Zhang F, Chen C, Zhang F, et al. Trichoderma harzianum containing 1-aminocyclopropane-1-carboxylate deaminase and chitinase improved growth and diminished adverse effect caused by Fusarium oxysporum in soybean. J Plant Physiol. 2017;210:84-94. doi:10.1016/j.jplph.2016.10.012
12. Melongenae OFS, Kareem HJ, Al-araji AM. Evaluation of Trichoderma Harzianum Biological Control Against Fusarium Fusarium رطفلا دض Trichoderma harzianum رطفلل ةيويحلا ةمواقملا مييقت oxysporum f . sp . Melongenae. 2017;58(4):2051-2060.
13. Zhang S, Sun F, Liu L, et al. Dragonfly-Associated Trichoderma harzianum QTYC77 Is Not only a Potential Biological Control Agent of Fusarium oxysporum f. sp. cucumerinum but Also a Source of New Antibacterial Agents. J Agric Food Chem. 2020;68(48):14161-14167. doi:10.1021/acs.jafc.0c05760
14. Michel C, Baran N, André L, Charron M, Joulian C. Side Effects of Pesticides and Metabolites in Groundwater: Impact on Denitrification. Front Microbiol. 2021;12. doi:10.3389/fmicb.2021.662727
15. Reyna PB, Albá ML, Rodríguez FA, et al. What does the freshwater clam, Corbicula largillierti, have to tell us about chlorothalonil effects? Ecotoxicol Environ Saf. 2021;208. doi:10.1016/j.ecoenv.2020.111603
16. Haas J, Zaworra M, Glaubitz J, et al. A toxicogenomics approach reveals characteristics supporting the honey bee (Apis mellifera L.) safety profile of the butenolide insecticide flupyradifurone. Ecotoxicol Environ Saf. 2021;217. doi:10.1016/j.ecoenv.2021.112247
17. Systemic Effects of the Pesticide Mancozeb – A Literature Review.
18. SIAMAK SB, ZHENG S. Banana Fusarium Wilt (Fusarium oxysporum f. sp. cubense) Control and Resistance, in the Context of Developing Wilt-resistant Bananas Within Sustainable Production Systems. Hortic Plant J. 2018;4(5):208-218. doi:10.1016/j.hpj.2018.08.001
19. Röhrich CR, Jaklitsch WM, Voglmayr H, et al. Front line defenders of the ecological niche! Screening the structural diversity of peptaibiotics from saprotrophic and fungicolous Trichoderma/Hypocrea species. Fungal Divers. 2014;69(1):117-146. doi:10.1007/s13225-013-0276-z
20. Gonzalez MF, Magdama F, Galarza L, Sosa D, Romero C. Evaluation of the sensitivity and synergistic effect of Trichoderma reesei and Mancozeb to inhibit under in vitro conditions the growth of Fusarium oxysporum. Commun Integr Biol. 2020;13(1):160-169. doi:10.1080/19420889.2020.1829267
21. Shang J, Liu B. Application of a microbial consortium improves the growth of Camellia sinensis and influences the indigenous rhizosphere bacterial communities. J Appl Microbiol. 2021;130(6):2029-2040. doi:10.1111/jam.14927
22. Jones JG, Korir RC, Walter TL, Everts KL. Reducing chlorothalonil use in fungicide spray programs for powdery mildew, anthracnose, and gummy stem blight in melons. Plant Dis. 2020;104(12):3213-3220. doi:10.1094/PDIS-04-20-0712-RE
23. Peláez-álvarez A, Santos-villalobos SDL, Yépez EA, Isela F, Reyna P cota. Synergistic effect of Trichoderma asperelleum T8A and captan 50® against Colletotrichum gloeosporioides (Penz.) Abigail. Rev Mex Ciencias Agrícolas. 2016;7(6):1401-1412.
24. Zin NA, Badaluddin NA. Biological functions of Trichoderma spp. for agriculture applications. Ann Agric Sci. 2020;65(2):168-178. doi:10.1016/j.aoas.2020.09.003
25. Suebrasri T, Somteds A, Harada H, et al. Novel endophytic fungi with fungicidal metabolites suppress sclerotium disease. Rhizosphere. 2020;16. doi:10.1016/j.rhisph.2020.100250
26. Indriyanti D, Rahmawati R, Widiatningrum T, Purwantoyo E. Effect of Trichoderma sp . secondary metabolite on the increase in leaf number of coconut plant Effect of Trichoderma sp . secondary metabolite on the increase in leaf number of coconut plant. J Phys. 2020;1567:7-11. doi:10.1088/1742-6596/1567/3/032040
27. Tian Y, Yu D, Liu N, Tang Y, Yan Z, Wu A. Confrontation assays and mycotoxin treatment reveal antagonistic activities of Trichoderma and the fate of Fusarium mycotoxins in microbial interaction. Environ Pollut. 2020;267. doi:10.1016/j.envpol.2020.115559
28. Hadibarata T, Syafiuddin A, Al-Dhabaan FA, Elshikh MS, Rubiyatno. Biodegradation of Mordant orange-1 using newly isolated strain Trichoderma harzianum RY44 and its metabolite appraisal. Bioprocess Biosyst Eng. 2018;41(5):621-632. doi:10.1007/s00449-018-1897-0
29. Saravanakumar K, Yu C, Dou K, Wang M, Li Y, Chen J. Synergistic effect of Trichoderma-derived antifungal metabolites and cell wall degrading enzymes on enhanced biocontrol of Fusarium oxysporum f. sp. cucumerinum. Biol Control. 2016;94:37-46. doi:10.1016/j.biocontrol.2015.12.001
30. Kumar T, Veena S, Karthikeyan S. Compatibility of Trichoderma asperellum with Fungicides, Insecticides, Inorganic fertilizers and Bio-pesticides Mushroom View project Production of tube rcrops View project. ResearchGate. Published online 2017. https://www.researchgate.net/publication/331519611
31. Vinale F, Sivasithamparam K, Ghisalberti EL, et al. Trichoderma Secondary Metabolites Active on Plants and Fungal Pathogens. Published online 2014:127-139.
32. Khan RAA, Najeeb S, Hussain S, Xie B, Li Y. Bioactive secondary metabolites from Trichoderma spp. Against phytopathogenic fungi. Microorganisms. 2020;8(6). doi:10.3390/microorganisms8060817
33. Tchameni SN, Cotârleț M, Ghinea IO, et al. Involvement of lytic enzymes and secondary metabolites produced by Trichoderma spp. in the biological control of Pythium myriotylum. Int Microbiol. 2020;23(2):179-188. doi:10.1007/s10123-019-00089-x
34. NapitupuluIlyas M, Kanti A, Im S. In vitro evaluation of Trichoderma harzianum strains for the control of Fusarium oxysporum f . sp . cubense. 2019;9(January):152-159. doi:10.5943/ppq/9/1/13
35. Stracquadanio C, Quiles JM, Meca G, Cacciola SO. Antifungal Activity of Bioactive Metabolites Produced by Trichodermaasperellum and Trichodermaatroviride in Liquid Medium. J fungi (Basel, Switzerland). 2020;6(4):1-18. doi:10.3390/jof6040263
36. Li N, Alfiky A, Wang W, Nourollahi K. Volatile Compound-Mediated Recognition and Inhibition Between Trichoderma Biocontrol Agents and Fusarium oxysporum. 2018;9(October):1-16. doi:10.3389/fmicb.2018.02614
37. Li MF, Li GH, Zhang KQ. Non-volatile metabolites from Trichoderma spp. Metabolites. 2019;9(3). doi:10.3390/metabo9030058
38. Rahman Khan M, Shahid S, Mohidin FA, Mustafa U. Interaction of Fusarium oxysporum f. sp. gladioli and Meloidogyne incognita on gladiolus cultivars and its management through corm treatment with biopesticides and pesticides. Biol Control. 2017;115:95-104. doi:10.1016/j.biocontrol.2017.09.010
39. Hu D, Yu S, Yu D, et al. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control. 2019;106. doi:10.1016/j.foodcont.2019.106748
40. Manganiello G, Sacco A, Ercolano MR, et al. Modulation of tomato response to rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front Microbiol. 2018;9(AUG). doi:10.3389/fmicb.2018.01966
41. Dini I, Marra R, Cavallo P, et al. Trichoderma strains and metabolites selectively increase the production of volatile organic compounds (Vocs) in olive trees. Metabolites. 2021;11(4). doi:10.3390/metabo11040213
42. Lombardi N, Salzano AM, Troise AD, et al. Effect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Profile of Strawberry Fruits. J Agric Food Chem. 2020;68(27):7246-7258. doi:10.1021/acs.jafc.0c01438
43. Contreras-Cornejo HA, Macías-Rodríguez L, Del-Val E, Larsen J. Full Title: Ecological Functions of Trichoderma Spp. and Their Secondary Metabolites in the Rhizosphere: Interactions with Plants Downloaded From.; 2016. http://femsec.oxfordjournals.org/
44. Kumari N, Srividhya S. Secondary metabolites and lytic tool box of trichoderma and their role in plant health. In: Molecular Aspects of Plant Beneficial Microbes in Agriculture. Elsevier; 2020:305-320. doi:10.1016/b978-0-12-818469-1.00025-0
45. Damodaran T, Rajan S, Muthukumar M, et al. Biological Management of Banana Fusarium Wilt Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Antagonistic Fungal Isolate CSR-T-3 (Trichoderma reesei). Front Microbiol. 2020;11. doi:10.3389/fmicb.2020.595845
46. Álvarez-García S, Mayo-Prieto S, Gutiérrez S, Casquero PA. Self-inhibitory activity of trichoderma soluble metabolites and their antifungal effects on fusarium oxysporum. J Fungi. 2020;6(3):1-11. doi:10.3390/jof6030176
47. Zhu F, Zhao X, Li J, Guo L, Bai L, Qi X. A new compound Trichomicin exerts antitumor activity through STAT3 signaling inhibition. Biomed Pharmacother. 2020;121. doi:10.1016/j.biopha.2019.109608
48. Redda ET, Mei J, Li M, Wu B, Jiang X. Biological Control of Soilborne Pathogens ( Fusarium oxysporum F. Sp. Cucumerinum) of Cucumber ( Cucumis sativus ) by Trichoderma sp. 2018;12:1-12. doi:10.17265/1934-7391/2018.01.001
49. Alamri SAM, Hashem M, Moustafa YS, Nafady NA, Abo-elyousr KAM. Biological control of root rot in lettuce caused by Exserohilum rostratum and Fusarium oxysporum via induction of the defense mechanism. Biol Control. 2018;7:1-3. doi:10.1016/j.biocontrol.2018.09.014
50. Ao N, Vi G. Evaluation of Antagonistic Effect of Trichoderma Harzianum against Fusarium oxysporum causal Agent of White Yam ( Dioscorearotundata poir ) Evaluation of Antagonistic Effect of Trichoderma Harzianum against Fusarium oxysporum causal Agent of White Yam ( D. 2018;(May). doi:10.19080/TTSR.2018.01.555554
51. Sinha A, Singh R, Verma A. Bioefficacy of Trichoderma harzianum and Trichoderma viride against Fusarium oxysporum f. sp. capsici causing wilt disease in chilli. ~ 965 ~ J Pharmacogn Phytochem. 2018;7(5). http://agriculture.gov.in
52. Chen S chen, Ren J jing, Zhao H jiao, et al. Trichoderma harzianum Improves Defense Against Fusarium oxysporum by Regulating ROS and RNS Metabolism , Redox Balance , and Energy Flow in Cucumber Roots. Phytopathology. 2019;109(6):972-982. doi:10.1094/PHYTO-09-18-0342-R
53. Frisvad JC. Safety of the fungal workhorses of industrial biotechnology : update on the mycotoxin and secondary metabolite potential of Aspergillus niger , Aspergillus oryzae , and Trichoderma reesei. Published online 2018:9481-9515.
54. Shenouda ML, Ambilika M, Cox RJ. Trichoderma reesei contains a biosynthetic gene cluster that encodes the Antifungal Agent Ilicicolin H. J Fungi. 2021;7(12):1034.
55. Yang Z, Qiao Y, Li J, Wu FG, Lin F. A Novel Water-Soluble Photosensitizer for Photodynamic Inactivation of Gram-Positive Bacteria. doi:10.1101/2020.05.29.124768
56. Watts R, Dahiya J, Chaudhary K, Tauro P. Isolation and characterization of a new antifungal metabolite of Trichoderma reesei. Plant Soil. 1988;107(1):81-84.
57. Pachauri S, Sherkhane PD, Mukherjee PK. Secondary Metabolism in Trichoderma: Chemo- and Geno-Diversity BT - Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications: Volume 2. Soil & Agroecosystems. In: Satyanarayana T, Das SK, Johri BN, eds. Springer Singapore; 2019:441-456. doi:10.1007/978-981-13-8487-5_17
58. Speckbacher V. Secondary Metabolites of Mycoparasitic Fungi. In: Raja SZERVESSS, ed. IntechOpen; 2018:Ch. 3. doi:10.5772/intechopen.75133
59. Mukherjee PK, Horwitz BA, Kenerley CM. Secondary metabolism in Trichoderma–a genomic perspective. Microbiology. 2012;158(1):35-45.
60. Marik T, Tyagi C, Balázs D, et al. Structural Diversity and Bioactivities of Peptaibol Compounds From the Longibrachiatum Clade of the Filamentous Fungal Genus Trichoderma . Front Microbiol . 2019;10. https://www.frontiersin.org/articles/10.3389/fmicb.2019.01434
61. Baiyee B, Pornsuriya C, Ito S ichi, Sunpapao A. Trichoderma spirale T76-1 displays biocontrol activity against leaf spot on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol Control. 2019;129:195-200. doi:https://doi.org/10.1016/j.biocontrol.2018.10.018
62. Abdel-Monaim MF, Abdel-Gaid MA, Zayan SA, Nassef DMT. Enhancement of Growth Parameters and Yield Components in Eggplant using Antagonism of Trichoderma spp. Against Fusarium Wilt Disease. Int J Phytopathol Vol 3, No 1 Int J Phytopathol. Published online 2014. doi:10.33687/phytopath.003.01.0510
63. Lombardi N, Salzano AM, Troise AD, et al. E ff ect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Pro fi le of Strawberry Fruits. Published online 2020. doi:10.1021/acs.jafc.0c01438
64. Gava CAT, Pinto JM. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol Control. 2016;97:13-20. doi:10.1016/j.biocontrol.2016.02.010
65. Yang C, Hamel C, Vujanovic V, Gan Y. Fungicide: Modes of Action and Possible Impact on Non-target Microorganisms. ISRN Ecol. 2011;2011:1-8. doi:10.5402/2011/130289
66. Gisi U, Sierotzki H. Fungicide modes of action and resistance in downy mildews. In: European Journal of Plant Pathology. Vol 122. ; 2008:157-167. doi:10.1007/s10658-008-9290-5
67. Contreras-Cornejo HA, Macías-Rodríguez L, Del-Val1 E, Larsen J. Ecological functions of Trichoderma spp . and their secondary metabolites in the rhizosphere : interactions with plants. FEMS. 2016;(February):1-17. doi:10.1093/femsec/fiw036
68. Macías-Rodríguez L, Contreras-cornejo HA, Adame-garnica SG, Larsen J. The interactions of Trichoderma at multiple trophic levels: inter- kingdom communication. Microbiol Res. Published online 2020. doi:10.1016/j.micres.2020.126552
69. Woo S, Fogliano V, Scala F, Lorito M. Synergism between fungal enzymes and bacterial antibiotics may enhance biocontrol. Published online 2002:353-356.
70. Whitmore L, Wallace BA. The Peptaibol Database: A database for sequences and structures of naturally occurring peptaibols. Nucleic Acids Res. 2004;32(DATABASE ISS.):593-594. doi:10.1093/nar/gkh077
71. Meng-Fei L, LGuo-Hong L, Ke-Qin Z. Non-Volatile Metabolites from Trichoderma spp. Metabolites. 2019;9(58). doi:10.3390/metabo9030058
72. Chen S chen, Zhao H jiao, Wang Z hong, Zheng C xia. Trichoderma harzianum -induced resistance against Fusarium oxysporum involves regulation of nuclear DNA content , cell viability and cell cycle-related genes expression in cucumber roots. Eur J Plant Pathol. Published online 2017:43-53. doi:10.1007/s10658-016-0978-7
73. Kumari I, Sharma S, Ahmed M. Tripartite Interactions between Plants, Trichoderma and the Pathogenic Fungi. INC; 2020. doi:10.1016/B978-0-12-818469-1.00032-8
Received: 2 January 2023/ Accepted: 130 March 2023 / Published:15 June 2023
Citation: Gonzalez MF; Galarza L; Valdez L, Quizpe G . A systematic review of antifungal activity of metabolites from trichoderma spp., and fungicides against Fusarium oxysporum. Revis Bionatura 2023;8 (2) 7. http://dx.doi.org/10.21931/RB/2023.08.02.7
