2023.08.02.75
Files > Volume 8 > Vol 8 No 2 2023
Bio-Synthesis
of zinc oxide nanoparticles and detect its antitumor activity against human
skin cancer cell line (A375).

Abid W
E1, Gdayea I A2 and Oraibi A G.3,*
1 Al-Iraqia
University, College of Education, Biology Department, Baghdad, Iraq.
2 Al-Iraqia University,
College of Education, Biology Department, Baghdad, Iraq.
3
Al-Nahrain
University, College of Biotechnology, Baghdad, Iraq.
*
Corresponding Email: [email protected]
Available from:
http://dx.doi.org/10.21931/RB/2023.08.02.75
ABSTRACT
In
this study, Lepidium sativum seeds were collected from the local markets
in Baghdad. Zinc oxide nanoparticles were manufactured from the aqueous extract
of Lepidium sativum by adding
zinc acetate in a green, safe and environmentally friendly manner. The
formation of zinc oxide nanoparticles was inferred by changing the color of the
extract from white to yellow, and for Detection of biosynthetic zinc oxide nanoparticles
Examinations were conducted to detect these nanoparticles, including diagnosis
using atomic force microscopy (AFM), which showed that the size of the
nanoparticles (13) nm and the surface roughness (80.51) nm compared with the
aqueous extract, which amounted to (23) nm and (57.22) nm respectively. The
diagnosis using UV rays showed a peak absorption of nanoparticles at 350 nm
compared with the aqueous extract, which reached 248 nm. As for the scanning
electron microscope (SEM) examination, the nanoparticles' size ranged between 46.97
- 81.07 nanometers, compared with the aqueous extract, which reached 676 -
591.8 nanometers. To determine the toxic or inhibitory effect against A375
cancer cells and HdFn normal skin cells, MTT cytotoxicity assay was performed
for aqueous extract and zinc oxide nanoparticles. The results showed that the
aqueous extract was effective against the cancerous cell line A375, as the
viability of the cells decreased with the increase in the concentration of the
aqueous extract. The IC50 ratio was equal to 140.0 mg/ml for the A375 cancer
cells, and the IC50 ratio was equal to 179.9 mg/ml for the normal HdFn cells. As
for zinc oxide nanoparticles, the effectiveness was more substantial than that
of the aqueous extract, and the vitality of cells was reduced in the cancerous
line A375. The normal line HdFn, the higher the concentration of nanoparticles,
and the IC50 ratio was equal to 59.46 mg/ml for the cancerous line. The IC50
ratio was equal to 196.9 mg/ml for the normal line.
Keywords:
Zinc oxide nanoparticles, Lepidium sativum, antitumor activity or
A375 cancer cells.
INTRODUCTION
Many plants have been used all or part of them in
traditional medicines for thousands of years. Still, without sufficient
scientific evidence, and since these materials contain effective natural
substances such as phenols, flavonoids and other substances, this may lead to developing
new natural drugs with low side effects or without effects 1. The
seeds of the plant Lepidium sativum, which belongs to the Brassicaceae family,
are substances used in folk remedies, as they are used in the treatment of many
diseases such as scurvy, asthma, and coughing. They are also used in treating
pimples and as a diuretic 2, 3. It was also found that
the active components in the cress seeds stimulate apoptosis in cancer cells,
thus killing cancer cells specifically without harming normal healthy cells. 4,5,6. Over the past few decades, nanotechnology
has been increasingly used in medicine, including applications for diagnosis,
treatment, and targeting of tumors more safely and effectively. Nanoparticle-based
drug delivery systems have shown many advantages in cancer treatment, such as
precise targeting of tumor cells, reduction of side effects and drug resistance
7,8. It has been suggested that nanoparticles can play a role in treating
cancer cells and the genes responsible for them 9. Studies on applying
NP drugs in immunotherapy and ablation therapy for cancer 10,11 also supported
this.
This nanoparticle-based drug delivery system is
believed to enhance immunotherapy 12. Many nanotherapeutic drugs
have been commercialized and introduced into the clinical stage in recent years.
A phase 1 clinical trial that used a targeted nanoparticle-based system to
deliver small interfering RNA (siRNA) in patients with cancer was conducted in
2010 13. NPs have been shown to provide platforms for combination
drug therapy and inhibit some drug resistance mechanisms, such as efflux
vectors on cell membranes 14. This was also supported by studies on
breast cancer (Alimoradi et al., 2018), ovarian cancer 15 and
prostate cancer 16.
MATERIAL AND METHODS
Plant sample collection
The seeds of the Lepidium sativum plant were collected
from the local markets in Baghdad during September 2021 and were confirmed by
the Seed Examination and Certification Department / Ministry of Agriculture.
Preparation of the plant aqueous extract of the
seeds of the Lepidium sativum plant
The seed Lepidium sativum plant extract was prepared
using the traditional method 18. By washing the seeds with water
well to remove the contaminants from the surface and soaking them for 30
minutes, then removing the moisture from them and drying them well with dry
air, 10 g of dried flaxseeds were ground and placed in a 250 ml glass beaker
containing 100 ml of deionized water, then The mixture was heated in a water
bath at 45 °C for 30 min, after which the extract was filtered using Whatman
No. 1 filter paper. The filtrate was stored at 4 °C for later use.
Manufacture of zinc oxide nanoparticles
Zinc oxide particles were prepared for the extract
of Lepidium sativum by melting 50 ml of aqueous plant extract at a temperature
of 40-45 °C, then 5 grams of zinc acetate were added to the solution with continuous
heating until it turned into a cohesive yellow paste. The paste was collected
in a glass Petri dish and dried at 45°C for 12 hours; then, the dried material
was ground to obtain a powder that was stored for detection, characterization
and other studies.
Detection and characterization of biosynthetic zinc
oxide nanoparticles.
The prepared samples were diagnosed and compared to
the aqueous extract using the following methods:
Atomic Force Microscopy (AFM)
Atomic force microscopy (AFM) assay was used to determine
the surface morphology of the as-prepared ZnO nanoparticles and their size and
diameter. A small drop of the sample solution, prepared in advance, was placed
on an 11x cm glass slide, left to dry at room temperature, and ready for testing
19.
Ultraviolet-Visibie spectrometer (UV)
1 ml of the aqueous extract of thyme seeds and zinc
oxide nanoparticles was taken and diluted with deionized water and then
centrifuged for 5 minutes in a centrifuge at a rate of 3000 rpm, then 1 ml was
taken and examined within the wavelength 200-1100 nm by a UV spectrometer according
to the method 20.
Energy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectroscopy (EDX) confirms
that the synthesis process produces pure nanoparticles 21.
Scanning Electron Microscopy (SEM)
The Japanese Meiji SEM scanner was used to determine
the shape and size of the particles in the prepared samples 22. Place
approximately 5 μl of ready-to-examine solutions onto a clamped gold-carbon
electron microscope holder, let them dry at room temperature, and test them
with different magnifying powers.
Cell lines
In this study, the natural skin cell line HdFn and
the cancer cell line A375 (melanoma) were used, which were obtained from the
University of Malaya/ College of Medicine/ Department of Pharmacy/ Center for
Natural Product Research and Drug Discovery/ Department of Malaysia
Pharmacology/ Faculty of Medicine University of Malaya Kuala Lumpur/Malaysia
The cancer line was maintained and developed. Tests were conducted on it at the
Biotechnology Research Center at Al-Nahrain University.
Sterilization methods:
Wet heat sterilization
The solid and liquid culture media and the used
solutions were sterilized by osmosis at a temperature of 121°C and at a
pressure of 15 pounds/ing2 for 15 minutes 23.
Dry heat sterilization
The needles used to grow and activate the bacteria until
they reached redness were sterilized by direct Bunsen flame, and the glassware
was sterilized for two hours at a temperature of 150 ° C in an electric oven 24.
Sterilization with chemicals
The culture room (Laminar air flow cabinet) was
sterilized with 70% ethyl alcohol and 30% chlorine.
Sterilization by filtration
The plant extracts and the heat-sensitive
filtration-prepared nanosolutions were sterilized using 0.22 µm osmotic
microfilters 24.
Preparation of solutions and reagents used in
tissue culture
Prepared solutions according to 25.
Phosphate buffer solution Phosphate – brine
This solution was prepared by dissolving 8 g of NaCl
salt, 0.2 g of KCL, 1.15 g of Na2H2PO4 and 0.2 g of Na2HPO4 in 900ml of
distilled water, and the pH was adjusted to 7.2. the use.
Trypsin Solution
The Preparation was prepared by dissolving 1g of
trypsin powder in 100 ml of PBS phosphate buffer solution and then sterilizing
it with a filter with holes with a diameter of 0.22 µm. Then it was divided into
10ml tubes and stored at -20°C.
Trypan Blue dye
The Preparation was made by dissolving 1 g of dye
powder in 100 ml of phosphate buffer solution to prepare a 1% solution; then,
it was filtered using a filter with holes with a diameter of 22.0 μm and kept
at a temperature of 4 °C until use.
EDTA solution
The Preparation was prepared by dissolving 1g of
Ethylene Diamine - Tetra Acetic Acid (EDTA) in 100 ml of PBS phosphate buffer
solution and sterilizing the solution by oxidation for 10 minutes. The
resulting solution was divided into 10ml doses in tubes and kept at 4°C until
use.
Trypsin - EDTA. Solution
The Preparation was prepared by mixing 20ml of
trypsin solution, 10ml of EDTA and 370ml of phosphate buffer PBS. The mixture was
kept at 4°C.
Serum Free Medium
It is a ready-made food medium (RPMI-1640)
extracted from fetal bovine serum.
Development and maintenance of the A375 and HdFn
cell lines.
The method (Freshney, 2015) followed the following
steps.
The cells were treated
individually using a water bath at a temperature of 37 °C.
The cells were placed in an animal cell
culture vessel with a diameter of 25 cm 2, which contained RPMI=1640 culture
medium on 10% bovine calf serum.
Incubating the container containing the cell
suspension as well as the culture medium in a CO2 5% incubator at a temperature
of 37 °C for 24 hours and after a day of incubation and after making sure that
the cell farm was growing and free from pollution, secondary farms were conducted
The cells were examined using an inverted
microscope to ensure their viability, contamination-free, and growth to the
required number (700-600) cells/ml.
The cells were transferred to the growth
cabin, and the used culture medium was disposed of.
The cells were washed with PBS solution and
discarded. The process was repeated twice for 15 minutes each time.
A sufficient amount of trypsin enzyme was
added to the cells, incubated for 60-30 seconds at 37 °C, and monitored until
they changed from a monolayer of cells to single cells. Then, the enzyme was
stopped by adding a new medium (BSA) Bovine Serum albumin-containing serum.
The cells were collected in centrifugal
tubes and placed in a centrifuge machine at a speed of 2000 rpm for 10 minutes
at room temperature to precipitate the cells and eliminate the trypsin and the
used culture medium.
The filtrate was discarded, and the cells
were suspended in fresh culture medium containing 10% serum.
Examination of the number of cells by taking
a specific volume of the cell suspension and adding the same volume of Trypan
Blue dye to determine the number of cells and their vitality percentage using a
Hemacytometer chip and according to the following equation
C= N×104×F/ml
whereas:
C: The number of cells in one ml of solution
F:
mitigating factor
N: The
number of cells in the slide
104: Slide dimensions.
The percentage of cell vitality in the
sample was calculated using a Hemacytometer chip according to the following equation:
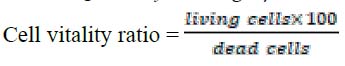
The cell suspension was distributed in new
containers and incubated in a CO2 5% incubator at 37°C for 24 hours.
2.6. MTT assay to
check cell viability after treatment with zinc oxide particles.
The values of samples IC50 were calculated using
the logarithmic dose inhibition curve or assay to determine the cytotoxic
effect of the nano-extract of the seeds of thyme plant on the cancer cell line
A375 and HdFn to use it in other assays High content screening (HCS).
The contents of the MTT examination kit.
MTT solution 1 ml × 10 glass vials
Solubilization solution 50 ml × 2 bottles
This method was carried out according to Rashid et
al. (2017).
The cancer cells were prepared (1 x 104-1
x 1106 x cell/ml-1) according to the section on cell development
(3-2-7-1), then flat base and covered. The dishes were gently covered with
sterile parafilm, stirred, and incubated in a 5% CO2 incubator at 37 °C for 24
hours. After incubation, the medium was removed.
After incubation, 100 µl of cell suspension was
added to each pit of the same plate.
The prepared concentrations of the aqueous extract
of turmeric seeds and zinc oxide nanoparticles (400, 200, 100, 50, 25, 12.5) μl
were added to the pits in the plate.
The plate was incubated for 24 hours at a
temperature of 37 °C.
10 μl of MTT solution was added to each hole at a 0.45
mg/ml concentration.
The plate was incubated for 4 hours at 37°C.
100 μl of solubilization solution was added to each
hole to dissolve Formazan crystals and then incubated for 5 minutes.
I was reading the absorbance of the sample at a
wavelength of 570 nm using an ELISA device (Bio-rad Germany) and at a
wavelength of 575 nm.
Statistical analysis of optical density readings to
calculate IC50 according to the following equation:

In the same way, the toxicity of zinc oxide
nanoparticles was investigated on the normal HdFn cell line.
RESULTS
.
Phenotypic characteristics of aqueous extracts of
flaxseed and biosynthesized zinc oxide nanoparticles.
In Figure 1, the color of the aqueous extract of
the plant appeared in white. It was noted that after adding zinc acetate
solution to the aqueous extract of the plant, the color of the extract turned
yellow. This color change is the first sign of the formation of nanoparticles made
of zinc oxide particles from plant leaf extract, where they manufactured zinc
nanoparticles in safe and environmentally friendly ways.

Figure
1. It
shows the color change of the aqueous extract during the formation of nanoparticles
Detection and characterization of zinc oxide
nanoparticles for the extract of the plant in comparison with the aqueous
extract:
Zinc oxide nanoparticles and aqueous extract were
diagnosed using atomic force microscopy (AFM).
The results of the AFM technique showed that the
zinc nanoparticles manufactured from the sage plant extract had different surface
topography measurements. Figure 2 shows that the size of the biosynthesized
nanoparticles was 13 nanometers, the size of the particles of the aqueous
extract of the plant extract was 23 nanometers, and the roughness rate of the
minute Nanoparticles was 80,51 nm, and the aqueous extract (57,22) nm. The
result of our research was consistent with the research line of Daphedar and
Taranath (2018). The size of ZnO NPs nanoparticles ranged between 10 and 85
nanometers.
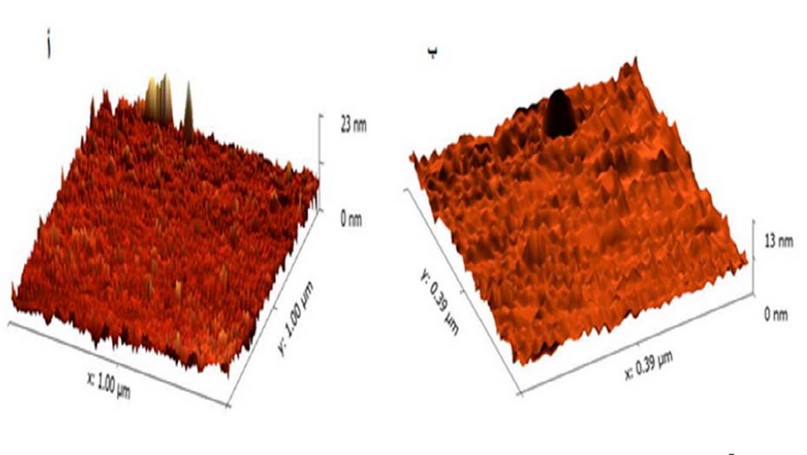
Figure
2. Diagnosing
biosynthetic nanoparticles using an atomic force test device, where A: aqueous
extract B: biomanufactured nanoparticles.
Diagnosis of nanoparticles and
aqueous extract using UV spectroscopy.
By using a spectrophotometer to
detect zinc oxide nanoparticles, it was shown in Figure 4-3 that the highest
absorption peak of nanoparticles at a wavelength ranged between 210-350 nm and
it is within the absorbance limits of zinc oxide nanoparticles, which confirms
the success of the biosynthesis process of zinc oxide particles.
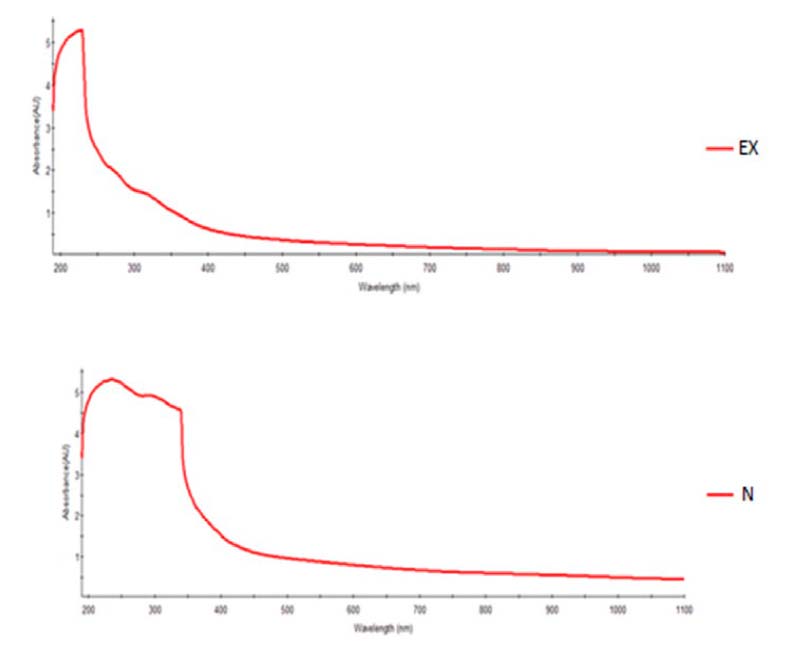
Figure
3. Characterization
of zinc oxide nanoparticles and aqueous extract using a UV absorber
Diagnosis of zinc oxide nanoparticles and
aqueous extract using X-ray Distributed Energy Spectrometry (EDX).
The results showed in Figure (4a) that the
components of the aqueous extract from the elements included C, Cl, O, Na, K,
S, Mg, Al, according to the weight ratios shown (21.7, 20.7, 18.6, 14.1, 11.2,
10.2, 1.8) sequentially, while the results in Figure 4b of the nano-extract
showed the appearance of the following elements (Zn, C, O, Ca) and in the
following weight ratios (44.9, 25.5, 25.1, 4.5) respectively.

Figure
4a,b.
Diagnostics of printed nanoparticles using EDX; A- Aqueous extract of currant
seeds; B- Biosynthesized ZnoNPs
Analysis of nanoparticle images and aqueous
extract using scanning electron microscopy (SEM).
It was shown in Figure 5a that the size of zinc
oxide nanoparticles ranged between 46.97 and 81.07 nanometers, and this agrees
with the accepted sizes of biosynthetic nanoparticles. The results in Figure 5b
showed that the size of the particles of the water extract of the aqueous
extract ranged between 591.8 and 676 nanometers, and this shows the clear difference
between the size of the nanoparticles and the size of the particles of the
water extract. It was scanning electron microscopy of ZnO NPs with a size
ranging between 62 and 94 nm 31.
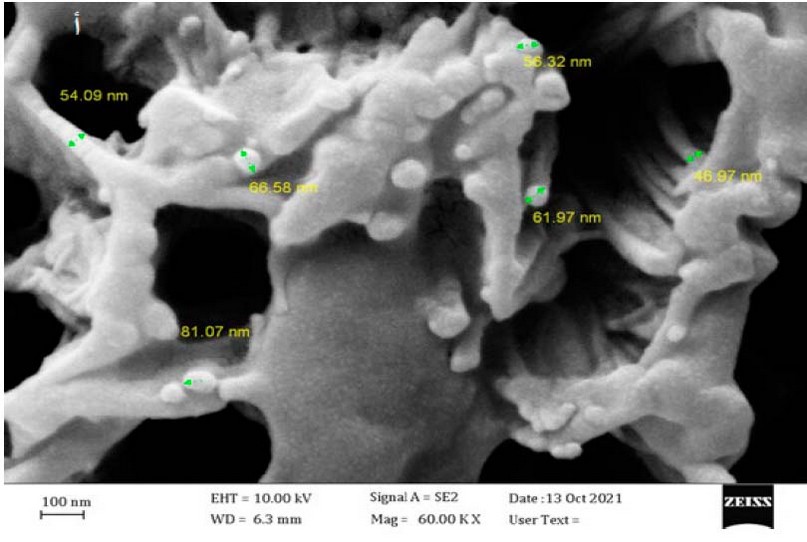
Figure 5a. Image showing the nano sizes
of zinc oxide nanoparticles by SEM

Figure
5b.
Image showing the nano sizes of the aqueous extract of Lepidium sativum
by SEM
MTT cytotoxicity test
The results of the test showed that the
nanoparticles showed effectiveness against A375 skin cancer cells, as the
viability of the cells was 52.83%, 63.70%, 77.62%, 90.59%, 95.95% at
concentrations of 400, 200, 100, 50 and 25, respectively.
At the same time, the nanoparticles showed cytotoxicity
on HdFn normal cells, as the cell viability was 66.47%, 74.46%, 90.97%, 94.64%,
95.29% at concentrations 400, 200, 100, 50, 25, respectively, as shown in Table
(1).
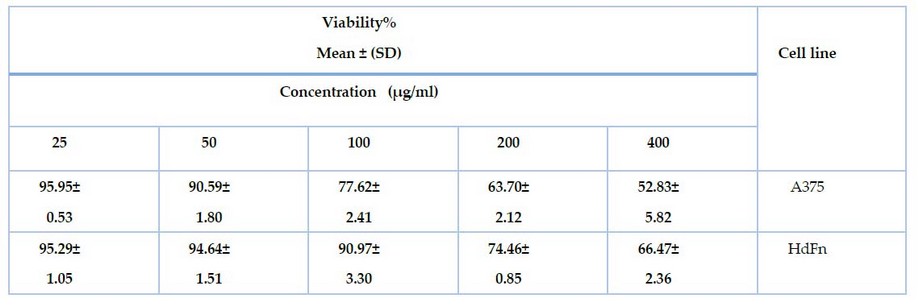
Table
1.
shows the effect of zinc oxide nanoparticles on cancer cell line A375 and
normal line HDFn using MTT assay.
In addition, the results showed significant
differences P ≤ 0.0001 when calculating IC 50 for both normal cells and cancer cells,
where the IC value of 50 for A375 cancer cells reached 129.3 µg/ml after being
treated with nanoparticles, and the IC value of 50 for normal cells HdFn 160.1 µg/ml,
as shown in Figure 6.

Figure 6. shows the IC50 values for the cancer cell line A375 and the normal
line HdFn when treated with zinc oxide nanoparticles.
Also,
the MTT toxicity assay was used to determine the toxic effect of the plant
extract on the skin cancer cell line A375 and the normal line HdFn. The results
showed that the aqueous plant extract showed effectiveness against the cell
line A375, as the cell viability was 54.78%, 61.92%, 75.81, 82.99, 85.03 at
concentrations 400, 200, 100, 50, and 25, respectively. At the same time, the
plant extract showed an effect on the HdFn cell line, as the cell viability
ranged by 70.80, 79.78, 89.35, 95.33, and 95.22 at concentrations 400, 200,
100, 50, 25 respectively, as shown in Table 2.
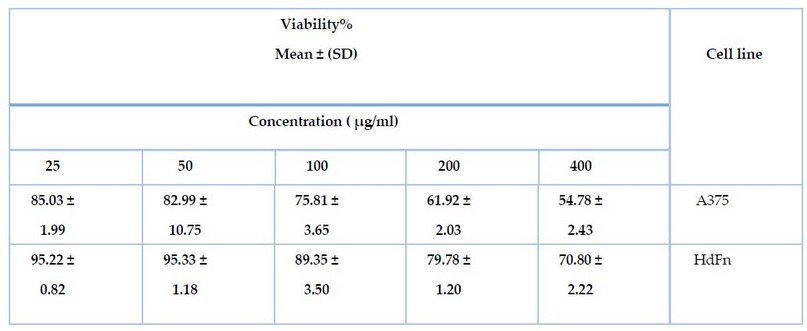
Table 2. shows the effect of the aqueous extract on the cancer cell line A375
and the normal line HdFn using the MTT test.
In addition, the results showed
significant differences P ≤ 0.0001 when calculating the IC50 when the plant extract
was treated with A375 cancer cells, the IC50 ratio was (140.0 µg/ml) and the
normal HdFn cells, the IC50 was equal to (179.7 µg/ml), as shown in Figure (7).
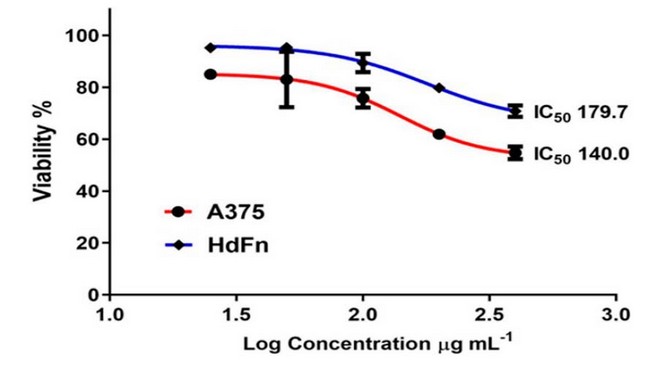
Figure
7.
shows the IC50 values for each cancer cell line A375 and the normal line HdFN
when treated with aqueous extract.
Several studies have confirmed the toxicity
of zinc oxide nanoparticles and their ability to inhibit many types of cancer,
with different levels of inhibition depending on the concentration of zinc
oxide nanoparticles and according to the type of cancer cell line used in the
study. Significant acid damage was also observed. Nuclear in cells treated with
the highest concentration of ZnO-NPs These results indicate that ZnO-NPs have
toxic and inhibitory potential in A375 cells and may induce oxidative stress.
To analyze the anticancer efficacy of ZnO
NPs, human hepatocellular carcinoma (HepG2) and normal murine hepatocytes
(hepatocytes) cells were used, and the cytotoxicity was determined using the
MTT assay. Concentrations up to 5 μg/ml have not been shown to lead to a
significant loss of cell viability. However, concentrations of 10-15 μg/ml are
beneficial. HepG2 vitality was reduced by 33%, while normal hepatocytes
remained unaffected 45.
DISCUSSION
This result was consistent with 27, who
synthesized zinc oxide particles from aloe vera and starch extract, and 28,
who synthesized zinc oxide particles from Lippia adoensis and 29. Another research path was the
size of ZnO NPs. In another research path, the size of ZnO NPs was 50-82 nm 39.
In another study, zinc oxide nanoparticles were synthesized from garlic skin extract.
The particle size was found to be about 25 nm, and the maximum surface particle
size of ZnO NPs was 23.34 nm for the study carried out by 32. Nanoparticles because the values between 300-380
nm are among the diagnostic properties of zinc oxide nanoparticles due to the
plasmon surface absorption, while the ultraviolet measurement showed that the
highest absorption peaks of the aqueous extract molecules were 248 nm. 33.
and 375 nm 34. While the absorption peak was close to our study by
researcher 35, it was 351 nm. This slight difference in the absorbance
values may be due to several factors, including the conditions and method of
manufacturing and the plant or its parts from which the nanoparticles were
manufactured. These results showed the disappearance of some elements
during the bio-manufacturing process of nanoparticles with the presence of zinc
at the highest weight percentage, and this is evidence of the formation of
nanoparticles and the entry of some influential groups and elements in the
aqueous extract into the manufacturing process. This result was within the
research line of 36. Whereas EDX analysis confirmed the presence of
O and Zn elements on the sample's surface, similar peaks were reported 37.
In a study by 38 the EDX of NPs revealed a precise formation of zinc
oxide nanoparticles where the atomic weight of oxygen was 48.83% while its previous
weight was 21%. On the other hand, the atomic weight of Zn was 37.16%, while
its last weight was 65.35%, while the other minor components present in ZnO-NPs
are due to the presence of ginger root extract. In another study, the diameter
of ZnO NPs was determined using SEM technique in the range of 20-80 nm 36.
In another line of research, the shape and size of zinc oxide nanoparticles
were determined by electronic scanning, and the SEM image showed zinc oxide
nanoparticles manufactured from Cassia alata leaf extract that most of the
nanoparticles are rod-formed within the diameter range of 30-50 nm. 40
This is consistent with our current study. The toxic effect of the aqueous extract on cancer cells may be due to secondary
metabolites; for example, phenolic compounds inhibit several enzymes
responsible for DNA replication in cancer cells, such as the enzyme
Topoisomerase I, II 41. Several studies have indicated that eating
food containing such compounds leads to a healthy lifespan and prevents heart
attacks and cancer 42. In a study compatible with ours, 43.
They revealed the toxic effects of ZnO-NPs on human melanoma A375 cells. The
results showed induction of apoptosis as confirmed by tests for chromosomal
condensation and caspase-3 activation. In a study, ZnO NPs were exposed to a
liver cancer cell line, and the results indicate that ZnO NPs stimulate
autophagy, downregulate the expression of caspase 3 and p53 markers, and
trigger apoptosis in HCC cells, thus limiting HCC cell growth and proliferation
44. In another study, ZnO nanorods were prepared with album Santalum
and dose-dependent cytotoxicity against MCF-7 cells was found. The results
revealed that ZnO nanorods induced apoptosis via an intrinsic mitochondrial
pathway dependent on caspase activation 46. Similarly, another study
using ZnO nanorods manufactured from Leea asiatica extract against MCF-7 cancer
cells reported similar results 47. ZnO NPs prepared from aerial
parts of Deverra Tortusa were evaluated against human colon cancer (Caco-2) and
A549 cell line. Through ROS stimulation, ZnO NPs showed cytotoxicity against
these two cell lines, while the human lung fibroblast line (WI38) did not show
significant cytotoxicity 48.
CONCLUSION
It can be concluded from
this study that the vitality of cells in the cancerous line and the normal line
decreases with increasing concentration of nanoparticles. As well as that the
viability of cells in the cancerous line and the normal line decreases with
increasing concentration of the aqueous extract.
REFERENCE
1
Mahassni, S and Al-Reemi, R.
Apoptosis and necrosis of human breast cancer cells by an aqueous garden cress
(Lepidium sativum) seeds extract. Journal of Biological Sciences. 2013;
20, 131-139.
2
Dugasani, S.L.; Balijepalli, M.K.
and Mallikarjuna Rao, P. Growth inhibition and induction of apoptosis in
estrogen receptor-positive and negative human breast carcinoma cells by
Adenocalymma alliaceum flowers. Current Trends in Biotechnology and Pharmacy.
2009; 3, 1–12.
3
Gokavi, S.S.; Malleshi, N.G. and
Guo, M. Chemical composition of garden cress (Lepidium sativum) seeds and its
fractions and use of bran as a functional ingredient. Plant Foods for Human
Nutrition (Formerly Qualitas Plantarum), 2005; 59,
105–111.
4
Das, S .,Tyagi, A and Kaur, H. Cancer modulation by glucosinolates:
a review Current science, 2000;79, pp. 1665-1671.
5
Divisi, D., Tommaso, S.D., Salvemini,
S., Garramone, M. and Crisci, R. Diet and cancer. Acta Biomedica, 2006;
77, pp. 118-123.
6
Diwakara, B.T., Duttaa, P.K.,
Lokeshb, B.R. and Naidu K.A. Bio-availability and metabolism of n-3
fatty acid rich garden cress (Lepidium sativum) seed oil in albino rats
Prostaglandins, Leukotrienes and Essential Fatty Acids, 2008; 78,
pp. 123-130.
7
Dadwal, A., Baldi, A., and Kumar Narang,
R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed.
Biotechnol. 2018; 46, 295–305.
8
Palazzolo, S., Bayda, S., Hadla,
M., Caligiuri, I., Corona, G., Toffoli, G. and Rizzolio, F. The clinical
translation of organic nanomaterials for cancer therapy focuses on polymeric
nanoparticles, micelles, liposomes and exosomes. Curr. Med. Chem. 2018;
25,4224–4268.
9
Chen, Y., Gao, D. Y., and Huang, L.
In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv.
Drug Deliv. Rev. 2015; 81, 128–141.
10
Riley, R. S., and Day, E. S. Gold
nanoparticle-mediated photothermal therapy: applications and opportunities for
multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.
2017; 9:e1449.
11
Yoon, H. Y., Selvan, S. T., Yang,
Y., Kim, M. J., Yi, D. K., Kwon, I. C. and Kim, K. Engineering nanoparticle
strategies for effective cancer immunotherapy. Biomaterials. 2018;
178, 597–607.
12
Zang, X., Zhao, X., Hu, H., Qiao,
M., Deng, Y., and Chen, D. Nanoparticles for tumor immunotherapy. Eur. J.
Pharm. Biopharm. 2017; 115, 243–256.
13
Davis, M. E., Zuckerman, J. E.,
Choi, C. H., Seligson, D., Tolcher, A., Alabi, C. A. Yan, Y., Heidel, J. and
Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted
nanoparticles. Nature, 2010; 464, 1067–1070.
14
Li, W., Zhang, H., Assaraf, Y. G.,
Zhao, K., Xu, X., Xie, J. Yang, D. and Chen, Z. Overcoming ABC
transporter-mediated multidrug resistance: molecular mechanisms and novel
therapeutic drug strategies. Drug Resist. Updat. 2016; 27,
14–29.
15
Alimoradi, H., Greish, K.,
Barzegar-Fallah, A., Alshaibani, L., and Pittalà, V. Nitric oxide-releasing
nanoparticles improve doxorubicin anticancer activity. Int. J. Nanomed. 2018;
13, 7771–7787.
16
Wang, H., Agarwal, P., Zhao, G., Ji,
G., Jewell, C., Fisher, J., Lu, X. and He, X. Overcoming Ovarian Cancer Drug
Resistance with a Cold Responsive Nanomaterial. ACS Cent. Sci. 2018b;
4, 567–581.
17
Zhang, J., Wang, L., You, X., Xian,
T., Wu, J., and Pang, J. Nanoparticle therapy for prostate cancer: overview and
perspectives. Curr. Top. Med. Chem. 2019; 19, 57–73.
18
Manokari, M.,
Ravindran, C. P. and
Shekhawat, M. S. Production of zinc
oxide nanoparticles using aqueous extracts of amedicinal plant Micrococca mercurialis
(L.) Benth. WSN, 2016; 30: 117-128.
19
Doaa, K. M. Synthesis and
characterization of silver nanoparticles and their antibacterial activity in
vitro. M.Sc. Thesis. Institute of Genetic Engineering and Biotechnology for
Post Graduate Studies, University of Baghdad. Baghdad. Iraq. 2015.
20
Bharathidasan, R. and
Panneerselvam, A. Biosynthesis and charactization of silver nanoparticles using
endphytic fungi Aspergillus concius, Penicilluim janthinellum and phomosis sp. J.B.S.R.
2012; 3:3`3163-3169.
21
Sham, S.N.; Ali, S.I.; Ali, S.R.;
Naeem, M.; Bibi, Y.; Ali, S.R.; Raza, S.M.; Khan, Y. and Sherwani, SK Synthesis
and Characterization of Zinc Oxide Nanoparticles for Antibacterial
Applications. Journal of Basic & Applied Sciences. 2016 , 12,
205-210.
22
Vanmathi Selvi, K. and Sivakumar,
T. Isolation and characterization of silver nanoparticles from Fusarium
oxysporum. Int. J Curr. Microbiol App .Sci., 2012; 1 : 56-
62.
23
Sultana, Y. Pharmaceutical
microbiology and biotechnology sterilization methods and principles. New Delhi-110062.
2007.
24
Ryan, K. and Ray, C. Sherris
medical microbiology 4th ed. Mc graw- hillNewYork. 2004.
25
Freshney, R.I. culture of animal
cell: a manual of basic technique and specialized applications, John Wiley and
sons. 2015.
26
Rashid H. Umamaheswari G. Evaluation
of the cytotoxic effect of ginger extract against prostate cancer modal using
in vitro study. W. J Pharm Scie. 2017; 6 (12): 1044-1053.
27
De O.primo, J., Bittencourt, C.,
Sierra-Castillo, A., Colomer, J., Jaerger, S., Teixeira, V. and Anaissi, F. Synthesis
of zinc oxide nanoparticles by ecofriendly routes: adsorbent for copper removal
from wastewater. Front. Chem. 2020; P. 1-13.
28
Demissie, M., Sabir, F., Edossa, G.
and Gonfa, B. Synthesis of zinc oxide nanoparticles using leaf extract of Lippia
adoensis (Koseret) and evaluation of its antibacterial activity. Journal of
Chemistry. 2020; p.1-9.
29
Santhoshkumar, J., Venkst, S.
and Rajeshkumar, S. Synthesis of zinc
oxide nanoparticles using plant leaf extract against urinary tract infection pathogen.
Resource- Efficient Technologies: 2017; Pages 459-465.
30
Daphedar and Taranath. Green
synthesis of zinc nanoparticles using leaf extract of Albizia saman (Jacq.)
Merr. and their effect on root meristems of Drimia indica (Roxb.) Jessop.
Journal homepage. 2018; Pages 93-102.
31
Gupta, M., Tomar, R., Kaushik, S.,
Mishra, R. and Sharma, D. Effective Antimicrobial Activity of Green ZnO Nano
Particles of Catharanthus roseus. Front Microbiol. 2018; 9:
2030.
32
Modi, S. and Fulekar, M. Green
Synthesis of Zinc Oxide nanoparticles using garlic skin extract and its characterization.
Journal nanstructures. 2020; Pages 20-27.
33
Mohammadian M., Es'haghi Z. and Hooshmand
S. Green and chemical synthesis of zinc oxide nanoparticles and size evaluation
by UV–vis spectroscopy. J. Nanomed. Res. 2018;7:175.
34
Ali, K., Dwivedi, S., Azam, A.,
Saquib, Q., Al-Said, M.S., Alkhedhairy, A.A. and Musarrat, J. "Aloe vera
extract functionalized zinc oxide nanoparticles as nanoantibiotics against
multi-drug resistant clinical bacterial isolates", J. Colloid Interface
Sci. 2016, 472, 145-156.
35
Vaishnav,j. Subha, V Kirubanandan,
S Arulmozhi, M Renganathan, S. Green Synthesis of Zinc Oxide Nanoparticles by
Celosia Argentea and Its Characterization, J. Optoelect. Biomed. Mater. 2017;
59-71. 9 (1) .
36
Aalami, A., Mesgari, M and Sahebker.
Synthesis and characterization of green zinc oxide nanoparticles with
antiproliferative effects through apoptosis induction and microRNA modulation
in breast cancer cells. Bioinorganic Chemistry and Applications. 2020;
p. 1-17.
37
Vanathi, P., Rajiv, P., Narendhran, S., Rajeshwari, S.,
Rahman, P. K. and Venckatesh, R. "Biosynthesis and characterization of
phyto mediated zinc oxide nanoparticles: a green chemistry approach," Materials
Letters, 2014; 134: 13–15.
38
Faisal, S., Jan, H., Shah, S.,
Shah, S., Khan, A., Akbar, M., Rizwan, M., Jan, F., Wajidullah., Akhtar, N.,
Khattak, A. and Syed, S. Green synthesis of zinc oxide (ZnO) nanoparticles
using Aqueous fruit extracts of myristica fragrans: their characterizations and
biological and environmental applications. Published by American Chemical
Society. ACS Omega 2021, 6, 14, 9709–9722.
39
Gupta, M., Tomar, R., Kaushik, S.,
Mishra, R. and Sharma, D. Effective Antimicrobial Activity of Green ZnO Nano
Particles of Catharanthus roseus. Front Microbiol. 2018; 9:
2030.
40
Sujatha, J., Asokan, S. and
Rajeshkumar, S. Antidermatophytic activity of green synthesized zinc oxide
nanoparticles using Cassia alata leaves.Jornal of Microbiology,
Biotechnology and Food Sciences. 2018; doi: 10.15414/jmbfs.2018.7.4.348-352.
41
Sameer, R., Nidhi, S., Tarun, V.,
Charan, S., & Jyoti, G. A review on naturally derived compounds for
potential anticancer activity Indian Journal of Drugs, 2016; 4
(3), 75-86.
42
Khan, N. and Mukhtar, H. Tea polyphenols
in promotion of human health Nutrients. J. Nutrients. 2019;11
(1), 39.
43
Alarifi, S., Ali, D., Verma, A.,
Alakhtani, S. and Ali, B. A. "Cytotoxicity and genotoxicity of copper
oxide nanoparticles in human skin keratinocytes cells," International Journal
of Toxicology, 2013; 32(4): 296–307.
44
Yang R., Wu R., Mei J., Hu F., Lei C. Zinc oxide
nanoparticles promotes liver cancer cell apoptosis through inducing autophagy
and promoting p53. Eur. Rev. Med. Pharmacol. Sci; 2021, 25:1557–1563.
45
Akhtar M.J., Ahamed M., Kumar S.,
Khan M.M., Ahmad J., Alrokayan S.A. Zinc oxide nanoparticles selectively induce
apoptosis in human cancer cells through reactive oxygen species. Int. J.
Nanomed; 2012, 7:845.
46
Kavithaa K., Paulpandi M., Ponraj
T., Murugan K., Sumathi S. Induction of intrinsic apoptotic pathway in human
breast cancer (MCF-7) cells through facile biosynthesized zinc oxide nanorods. Karbala
Int. J. Mod. Sci; 2016, 2:46–55.
47
Ali S., Sudha K.G., Karunakaran G.,
Kowsalya M., Kolesnikov E., Rajeshkumar M.P. Green synthesis of stable
antioxidant, anticancer and photocatalytic activity of zinc oxide nanorods from
Leea asiatica leaf. J. Biotechnol; 2021,329:65–79.
48
Selim Y.A., Azb M.A., Ragab I., Abd
El-Azim M.H. Green synthesis of zinc oxide nanoparticles using aqueous extract
of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020;10:3445.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published:
June 15, 2023
Citation: Abid W E, Gdayea I A, Oraibi A G. Bio-Synthesis of zinc oxide
nanoparticles and detect its antitumor activity against human skin cancer cell
line (A375). Revis Bionatura 2023;8 (2) 75. http://dx.doi.org/10.21931/RB/2023.08.02.75