2022.07.03.23
Files > Volume 7 > Vol 7 No 3 2022
Purification and characterization of glutaminase from Iraqi fruit of Capsicum annuum
1 Department of Molecular and Medical Biotechnology, College of Biotechnology,
Al-Nahrain University, Baghdad, Iraq.
* Correspondence: [email protected] ; Tel.: (009647712863493)
Available from: http://dx.doi.org/10.21931/RB/2022.07.03.23
ABSTRACT
L-glutaminase has recently attracted much attention due to its medicinal and industrial potential. It's an antileukemic drug with flavor-enhancing properties when used in fermented foods manufacturing. The enzyme purification was determined by ammonium sulfate precipitation, Ion exchange chromatography and gel filtration chromatography with estimating glutaminase activity and characterization of purified glutaminase in several parameters such as substrate concentration and reaction time pH, and temperature influence in this study. As a result, glutaminase is purified in three steps: ammonium sulfate precipitation with 30% saturation, DEAE-Cellulose and Sephacryl S-200. The specific activity increased to 85 U/mg protein with 4 folds of purification and 18% enzyme recovery. When glutaminase was incubated in the presence of 150 millimolar glutamine at thirty-five centigrade for thirty minutes, the enzyme reached its maximal activity of 1.8 u/ml, in the presence of a 0.05 Molar potassium phosphate buffer solution, at pH 8.
Keywords: Capsicum annum, glutaminase, enzyme activity, glutaminase purification
INTRODUCTION
Enzymes have a critical role in preserving and maintaining financial, commercial, and industrial products since they are crucial in several biological and non-biological processes. They cover all metabolic responses and increase the response rate without affecting the end product 1. Glutaminase is a hydrolytic enzyme that breaks down Lglutamine into L-glutamic acid and ammonium ions. It is essential for nitrogen metabolism2.
Because of its medicinal and commercial implications, L-glutaminase has gotten much attention. Due to its accelerated hydrolysis conversion of glutamine to glutamic acid and ammonium, glutaminase is employed in the food service sector as a taste enhancer that raises glutamic acid content3,4. The therapeutic and industrial applications of L-glutaminase have received much interest. It's an antileukemic drug with flavor-enhancing properties when used in fermented foods manufacturing. Microorganisms (such as fungus and bacteria), animal tissues and Plants all have glutaminase activity. Gawlik-Dziki5 employed it in cancer enzyme treatment, particularly for acute lymphocytic leukemia.
Glutaminase is also utilized to flavor foods, particularly in the sauce of soy and other Asian industry. Microbial glutaminase has found new applications in clinical analysis and even metabolite manufacturing hence advances in biotechnology's utilized in biosensors to detect glutamine quantities in human and hybridoma cell cultures without needing to measure glutamic acid separately6. Submerged fermentation (SmF) is used to produce glutaminase for commercial purposes. SSF has also proven to be a viable strategy for creating a variety of bioprocesses, including the large-scale production of industrial enzymes4 Green pepper fruits are one of the world's most extensively consumed veggies. Pepper fruits are renowned for their vitamin C and A content and their mineral content. As a result, a daily fruit intake of 60–80 g can deliver a hundred percent and twenty-five percent of the daily vitamin A and C requirements.
Furthermore, this horticulturist's creation has considerable amounts of antioxidant compounds beneficial to one's health., including carotenoids, flavonoids, and other polyphenols, among others 7. When compared to microorganisms, plant sources are natural and readily available sources of enzymes. They don't pose any risks and require less caution when handling 8. As a result, using plant sources for enzyme extraction is safe and straightforward. So the primary goal of this research is to look into the production of capsicum annum and establish its L-glutaminase activity and purification, then characterization it.
MATERIALS AND METHODS
Crude Extract.
Glutaminase extracted According to Barbaree 9; glutaminase was isolated from the plant by homogenizing 20 g of plant material in six volumes of sodium phosphate buffer 0.05M, pH 8, including sodium chloride 1.5 M, PMSF 1 mM, EDTA 1 mM, and glycerol 10% (w/v), and centrifuging for twenty minutes at 1000 revolutions per minute. The supernatant was thought to contain a raw enzyme.
Purification
This step was accomplished by ammonium sulfate precipitation. Ammonium sulfate was added to the extract enzyme liquid with gradual saturation ratios ranging between 20% to 80%. The precipitate of each saturation ratio was dissolved in a suitable volume of 0.02 M phosphate buffer at pH7. The sample was dialyzed overnight at 40C against phosphate buffer. Dialyzed sample concentrated on sucrose and stored at 4 0 C 10.
DEAE-Cellulose
Column of DEAE-cellulose (19 x 1.9 cm) was equilibrated with 20mM Tris-HCl buffer, pH 7.0, before being loaded with the concentrated crude extract. Different concentrations of NaCl prepared in the same buffer at a 1 mL/min flow rate were used to elute the enzyme, and 3mL fractions were collected.
Sephacryl S-200.
Glutaminase (peak with maximum activity) was applied to a Sephacryl S-200 column (88 x 0.7cm) that had been pre-equilibrated with the same buffer at a flow rate of 0.5mL/min, and 3mL fractions were collected.
Enzyme Assay
Assay for enzyme According to13, glutaminase was determined using the Nesslerization method, which involves the conversion of L-glutamine to Ammonia and L-glutamate.
Protein concentrations in crude extracts of plant and enzyme concentration were assayed using Bradford's technique 11.
Characterizations of glutaminase
Bello et al. 12 investigated the Influence of several variables on purified glutaminase activity.
Effect of glutamine concentration
Glutaminase activity was determined by incubating it with various glutamine concentrations (25,50, 100, 150, 200, and 250mM), and then the level of activity was established using Novak and Philips' method 13.
Effect of reaction time
The enzyme activity of the purified enzyme was measured after different incubation durations (15, 30, 45, 60, and ninety minutes) at thirty-seven centigrade.
Effect of pH on activity and stability of glutaminase
The optimum pH for maximum glutaminase activity was determined by performing an enzyme activity assay at different pH values ranging from 7.5 to 9. For that purpose, 1 % glutamine was prepared by the 0.02 M phosphate buffer (pH 7.5 to 9). Reaction mixtures were incubated at 35°C for 30 min, and the activity of glutaminase was measured as described above. The effect of pH on glutaminase stability was determined by pre-incubating the enzyme without substrate at different pH values (7.5 to 9) using different phosphate buffer pH. The residual glutaminase activity was determined according to the assay procedure.
Effect of temperatures on activity and stability of glutaminase
The effect of temperature on the glutaminase activity was determined by incubating the reaction mixtures at different temperatures ranging from 25 to 40°C. After incubation, glutaminase activity was assayed. Pre-incubating the glutaminase enzyme without substrate at various temperatures was used to examine the Influence of temperature on glutaminase stability (25, 30, 35 and 40). The residual glutaminase activity was determined according to the assay procedure
RESULTS
Several purification procedures were set up to get purified enzymes for further characterization research. This study used seven percentages of saturation ratios to achieve ammonium sulfate precipitation. Figure (1) shows that as the ammonium sulfate precipitation ratio increased, the specific activity of the enzyme dropped, with the optimal value being 30%.
Table 1 summarizes the findings of glutaminase purification steps. The chromatography elution profile on the DEAE-cellulose column figure (2) revealed two peaks of proteins. Peak two, which had the highest glutaminase activity, was loaded onto a Sephacryl S-200 column. As shown in figure (2), one protein peak occurred during the washing stage, and another peak appeared following elution with sodium chloride gradient concentrations.
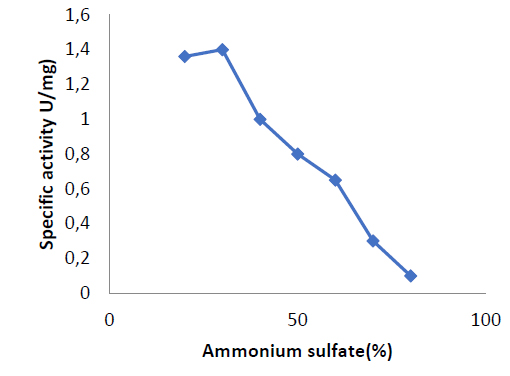
Figure 1. precipitation of enzyme at different ratios of ammonium sulfate saturations
The absorbance at 280 nm of each eluted fraction was used to detect these two protein peaks. The activity of glutaminase was measured in the second protein peak. The second peak eluted in fractions 37 to 51 had enzyme activity of 1.5, according to the results shown in figure (2).
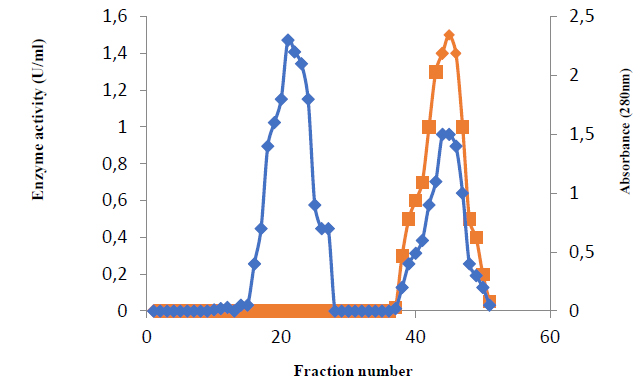
Figure 2. Ion exchange chromatography(Column of DEAE-cellulose (19 x 1.9 cm) was equilibrated with 20mM Tris-HCl buffer, pH 7.0, flow rate 1 ml/min.
After elution with phosphate buffer, just one peak (fractions 16 to 28) representing glutaminase activity appeared, and enzyme activity reached 1.8 u/ml, as shown in figure (3).
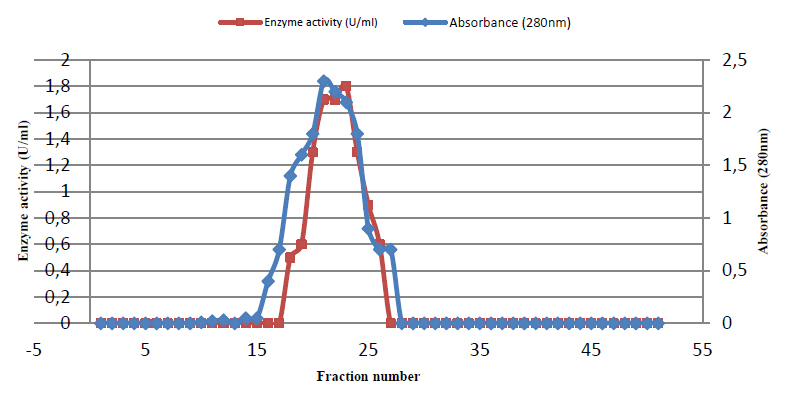
Figure 3. Gel filtration of glutaminase Sephacryl S-200 column (88 × 1.6 cm) equilibrated with the same buffer at a flow rate of 0.5mL/min, and 3mL fractions were collected.
Finally, the protein concentration, enzyme activity, and specific activity in this concentrate were 0.04,1.8, and 45, respectively, with a purification fold of 4 and an enzyme yield of 200 percent, as shown in table (1).
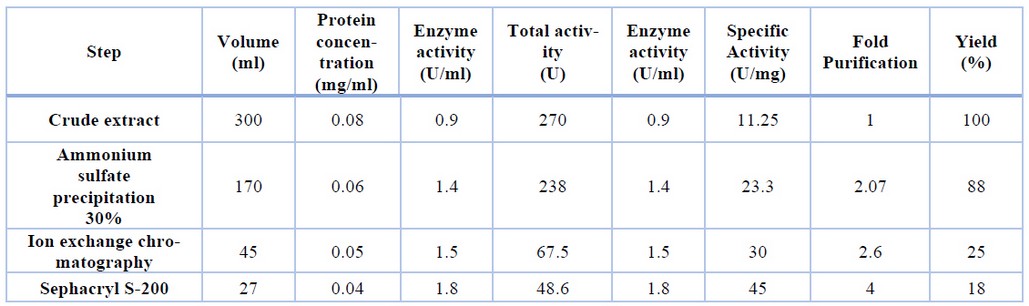
Table-1. Purification steps of glutaminase from Capsicum annum
Characterization Of Glutaminase
The concentration of the substrate
The glutaminase activity rose progressively as the content of L-glutamine increased, according to the results shown in figure (4). When the substrate concentration was 150mM, glutaminase activity was 1.8 U/ml; Activity of Glutaminase was 1.8 U/ml at this concentration

Figure 4. Optimum glutamin (substrate) concentration
Reaction time has an effect
The ideal reaction time was 30 minutes, and the enzyme activity was 2 units per milliliter, as shown in figure (5).
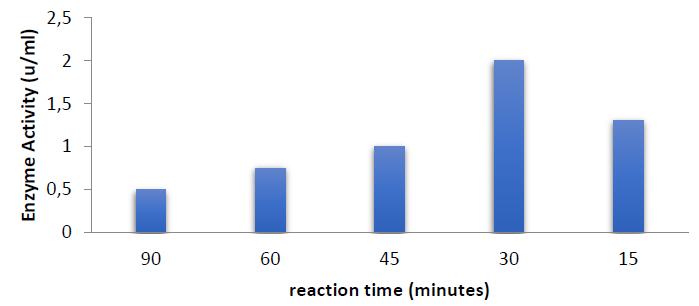
Figure 5. Optimum catalytic reaction time
The effect of pH
The effect of pH on Capsicum annuum glutaminase activity was examined. When the pH of the reaction mixture was increased to 8.0, the highest glutaminase activity was found, and the activity of the enzyme was three units per milliliter at this pH, as in figure (6). Glutaminase was more stable and kept all its activity at pH 8, as illustrated in figure (7).
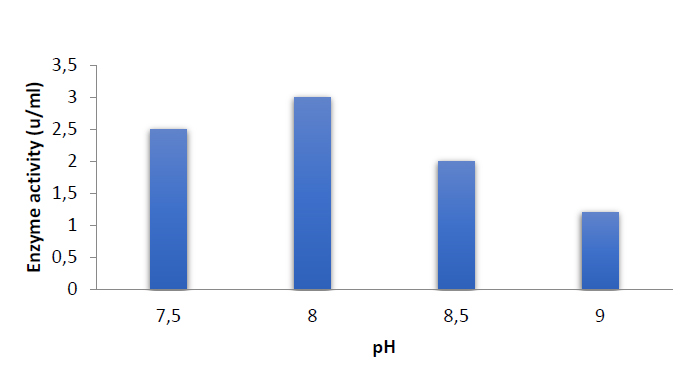
Figure 6. Effect of pH on Enzyme activity
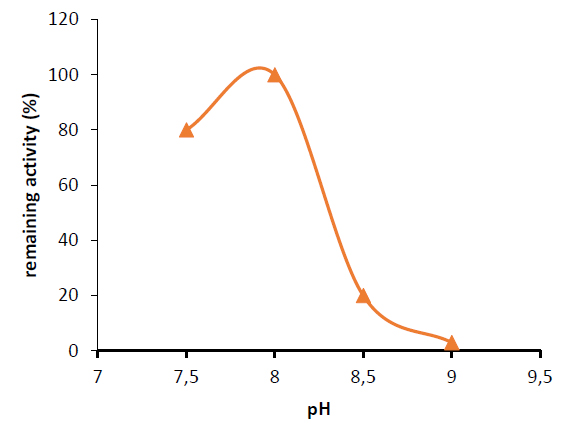
Figure 7. Effect of pH on enzyme stability
Temperature's Influence
Different temperatures (25, 30, 35, and 40°C) were utilized to find the optimum enzymatic reaction temperature for the activity of glutaminase isolated from the Capsicum annum.
When the temperature of the reaction mixture was elevated to 35°C, the highest activity of glutaminase was achieved, as shown in figure (8). At this temperature, the enzyme activity was increased to 5 Units per ml. The highest glutaminase activity in most species was discovered to be at 37°C 2.

Figure 8. The best temperature for glutaminase activity
Increases or decreases in the temperature of the incubation chamber above or below the ideal temperature, on the other hand, result in a drop in enzyme activity.
These fluctuations in asparaginase activity revealed that the optimal temperature for maximum activity of the asparaginase reaction was 37°C, with activity decreasing when the temperature was above or below the optimum. A nonlinear relation between glutaminase and temperature stability was detected in figure (9).
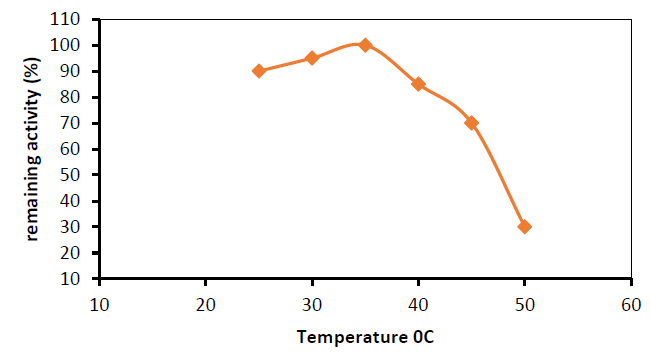
Figure 9. Stability of purified glutaminase at different temperatures
DISCUSSION
These findings were confirmed by14, who discovered that enzyme activity and substrate concentration have a positive connection and that when the asparaginase concentration was constant, the reaction rate increased with increasing substrate concentration until the maximum rate was reached (steady-state), after which the increase in substrate concentration had no effect on the rate of reaction and had no significant effect. There are no enzyme molecules in a steady state. Free to act on molecules that aren't substrates. Furthermore, substrate inhibition might develop when there is an excessive amount of substrate in the reaction mixture; substrate inhibition might develop15, according to Figure (4). When the glutamine concentration was 150mM, glutaminase generated by the Capsicum annuum attained a steady state.
According to previous findings, glutaminase binds substrate properly in the reaction mixture after 30 minutes of incubation, resulting in maximum enzyme activity. According to to16, time is an essential factor in determining enzyme activity; hence methods with short incubation durations are preferred when evaluating enzyme activity.
Most plant asparaginases have been found to have their highest activity at alkaline pH ranges, such as 7.5, 8.0, and 8.517. Most plants have their highest enzyme activity at or near neutral pH5. The most significant activity of E. coli asparaginase was observed at alkaline pH, which is most likely related to aspartic acid and aspartate balance. Between pH 7.5 and 8, moreover half of its activity was preserved. Although maximum activity at physiological pH is one of the requirements for anticancer activity in L-asparaginase, the purified enzyme would be helpful because it kept 80 percent of its activity at pH 7.5. The range of temperature was selected to study its Influence and was shown to be the most conducive to enzyme activity13. When incubated for up to 24 hours at pH 8, the enzyme showed stability, retaining 100% of its initial activity (7). The pH optimum of L-asparaginases from various plants, on the other hand, ranged from 8.0 to 8.5 15.
L-aspartic acid has a stronger preference for the enzyme's active site at an acidic pH. pH fluctuations are caused by changes in the concentration of hydrogen ions (H+) in the reaction mixture; This can lead to significant alterations in the protein's three-dimenttional structure, resulting in enzyme destruction18. The Influence of pH on enzyme activity, on the other hand, was caused by its effect on the ionization state of the substrate7. In these circumstances, It acts as a competitive deterrent. In an alkaline pH environment, the equilibrium swings in favor of glutamate. Which has a lower affinity for the active site enabling; in this instance, the interaction with the substrate L-glutamine is more advantageous19,20. In this situation, the link with the substrate L-glutamine is well balanced16. Bello12 discovered that polyphenol oxidase from Irvingia gabonnensis works best at pH 8.0.
Up to 180 minutes of incubation time was tested on the crude extract of chitosanase activity from pepper leaves and Opuntia peels. The chitosanase activity from pepper leaves was found to rise as the reaction time increased up to 180 minutes at 40°C. The activity of chitosanase and the reaction time are proportional. Similarly, chitosanase from opuntia peels increased as the reaction time was extended up to 120 minutes at 40°C 11. After 1 hour of incubation at 37°C, the enzyme activity remained steady. After incubation for 15 minutes20, asparaginase from Vigna. unguiculata was stable up to 40°C. After incubation at 40°C and 450°C, asparaginases from Pectobacterium carotovorum and Capsicum annuum kept their initial activity for 60min, respectively, 21
CONCLUSIONS
Glutaminase was purified in three steps; first 30% saturation of ammonium sulfate precipitation, second DEAE-Cellulose chromatography, and sephacryl S-200 chromatography. When glutaminase was incubated for 30 minutes at 35°C in the presence of 0.05 molar potassium phosphate buffer solution at pH 7, it was incubated with one hundred fifty mili molar glutamine, the enzyme's maximum activity was 1.8 unit per ml.
REFERENCES
1. Abdallah, N.A; Amer, S.K. and Habeeb, M.K." Screening of L-glutaminase produced by actinomycetes isolated from different soils in Egypt". Int J ChemTech R 2012,4: 1451–1460.
2. Abdel Hameed, A.M. "Production And Characterization Of L-Asparaginase From Local Isolate Of Serratia Marcescens Bacteria".M.Sc. thesis, College of Science, University of Baghdad, Iraq.2005.
3. Ren, J.; He, F.; and Zhang, L;. "The construction and application of a new PPY-MSPQC for L-asparaginase activity assay". Sensors and Actuators. J. 2010, 145: 272-27.
4. Yokatsuke, T.; "Fermented Protein Foods in the Orient", with Emphasis on Shoy and Miso in Japan. In : Microbiology of Fermented Foods. (ed. Wood B J B). Elsevier Applied Science, London, UK,1985. 197:247.1985.
5. Gawlik-Dziki, U.; Z³otek, U.; and Swieca, M;. "Characterization of Polyphenol oxidase from butter lettuce" (Luctuca sativa var.Capitata L.). Food Chem. J. 2007, 107: 129-135.
6. Sabu, A.; Keerth, T. R.; Rajeev,S;. and Kandersekran, M;.“ L;-Glutaminase production by marine Beaueria sp. under solidstate fermentation.Process Biochemistry ,2000.35 : 705–710.
7. Bull, A.; and Bushnel. M. E.; "Environmental control of fungal growth. In : The filamentous fungi" (eds. Smith J E and Berry D E). 1993. 2P: 1-26.Edward Arnold, London.
8. Barbaree, M. J.; and Harless, E. J.; Why bacteria are not enzymes and other essentials? .National trade Publications, Atlanta.1995.
9. Chang, K. S.; and Farnden, K. J.; Purification and properties of asparaginase from Lupinus arboreus and Lupinus angustifolius. Arch Biochem Biophys.. 1981, Apr 15;208(1):49-58.
10. Oza, V. P.; "Isolation And Characterization Of L-Asparaginase From Plant Species Of Olanaceae And Fabaceae". Ph. D. thesis.College of Science, Sardar University. India. 2009.
11. Bradford, M.M.; "A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding". Annal. Biochem. J. 1976, 72: 248-254.
12. Bello, A. B.; Sule; M. S.; and Al-Hassan, A. J.; "Optimum pH and pH stability of crude olyphenol Oxidase (PPO) extracted from five fruit samples commonly consumed in Kano State in Nigeria". Bayero J. Pure and Appl. Sci. 2011. 4(1), 26-31.
13. Novak, E. K. and Philips, A. W.; "Glutamine as a substrate for l-asparaginase from Serratia marcescens". J Bacteriol. 1964. 117, 593–600.1964.
14. Al-Noab, R. 2005. "Enzyme Conception". Arabic science. network.www.olom.info.
15. Martinek , R.; "Practical Clinical Enzymol". J. Am. Med. Tech. 1969, pp:31-162.
16. Dalaly, . B. K.; "Extraction And Purification Of Enzymes. In: Understanding Of Enzyme". Al-Mosel University Press, Al-Mosel.1990, pp:1-469.
17. Khalaf, Z. A.; Al-Ani, N. K.; and Jasim, H. M.; "Optimum conditions for asparaginase extraction from Pisum sativum subspp Jof," Iran Journal of Plant Physiology,2012. 2:. 517–521,
18. Tortora, G.J. ;Funke, B. R.; and. Case, C.L.; Microbiology. 8th ed.Pearson Education, Inc. San Francisco. 2004. New York.
19. Miller, M.; Mohana, R. J.K;. Wlodawer A.; and Gribskov, M.R;. "Crystal structure of Erwina chrysanthemi L-asparaginase with bound L-aspartate. 1993". FEBS Lett. J. 328(3), 275-279.
20. Lubkowski, J;. Wlodawer, A.; Ammon, H. L;.Copeland, T. D.; and Swain, A. L;. "Structural Characterization Of Pseudomonas 7a Glutaminase-Asparaginase". Biochem. J. 1994, 33, 10257- 10265.
21. Kumar, s.; Venkata Dasu V.; and. Pakshirajan, K.; "Purification and characterization of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428," Bioresource Technology,2011. 102: 2077–2082.
Received: 29 March 2022 / Accepted: 12 April 2022 / Published:15 Agoust 2022
Citation: Suhail Zbar N. Purification and characterization of glutaminase from Iraqi fruit of Capsicum annuum. Revis Bionatura 2022;7(3) 23. http://dx.doi.org/10.21931/RB/2022.07.03.2
